- Original Link
- Updated on May 14, 2022
- Authors:
- Jozef Bartunek (Cardiovascular Center, OLV Hospital Aalst, Belgium)
- Anthony Mathur (Centre for Cardiovascular Medicine and Devices, William Harvey Research Institute, Queen Mary University of London, London, UK)
- Marc Vanderheyden (Cardiovascular Center, OLV Hospital Aalst, Belgium)
- John Buckley (Cardiovascular Center, OLV Hospital Aalst, Belgium)
全文摘要
这篇文章是一篇关于心肌生物制剂递送技术的学术论文,主要介绍了心肌生物治疗的目的、临床试验的挑战、细胞保留的问题、不同细胞递送技术以及未来发展方向等。
以下是对这些核心内容的简要概述:
- 心肌生物治疗的目的:
旨在通过干细胞等生物方法促进心肌结构和功能修复,阻止或逆转导致心力衰竭进展的事件。 - 临床试验的挑战:
- 尽管细胞治疗在概念上是合理的,但在不同患者群体中的整体结构和功能益处有限。
- 存在患者特异性变量和细胞处理等因素影响疗效。
- 细胞保留的问题:
- 细胞保留于目标区域的比例通常不超过 5-10%,许多细胞最终停留在远离心脏的器官中。
- 临床研究表明,注射标记的干细胞后,心肌信号会逐渐下降。
- 细胞递送技术:
- 经皮冠状动脉细胞递送:通过冠状动脉进行细胞转移,是 STEMI 患者的首选方法。
- 逆行冠状静脉注射:通过冠状静脉系统进行的实验模型,理论上比直接冠状动脉或心肌内注射更安全。
- 经皮心内膜下心肌细胞递送:在缺血性心力衰竭或慢性心肌缺血的情况下,直接心内膜下注射是首选的细胞递送方式。
- 生物治疗的未来发展:
- 包括对心肌微环境 - 细胞和器械 - 微环境相互作用的更好理解。
- 通过数学建模和实验测试来验证各种针头设计,以提高细胞留存率。
这篇文章为心肌生物制剂递送技术的发展提供了详细的策略和技术分析,并通过探讨不同递送方法的优缺点,展示了未来提高细胞留存率和存活率的可能途径。
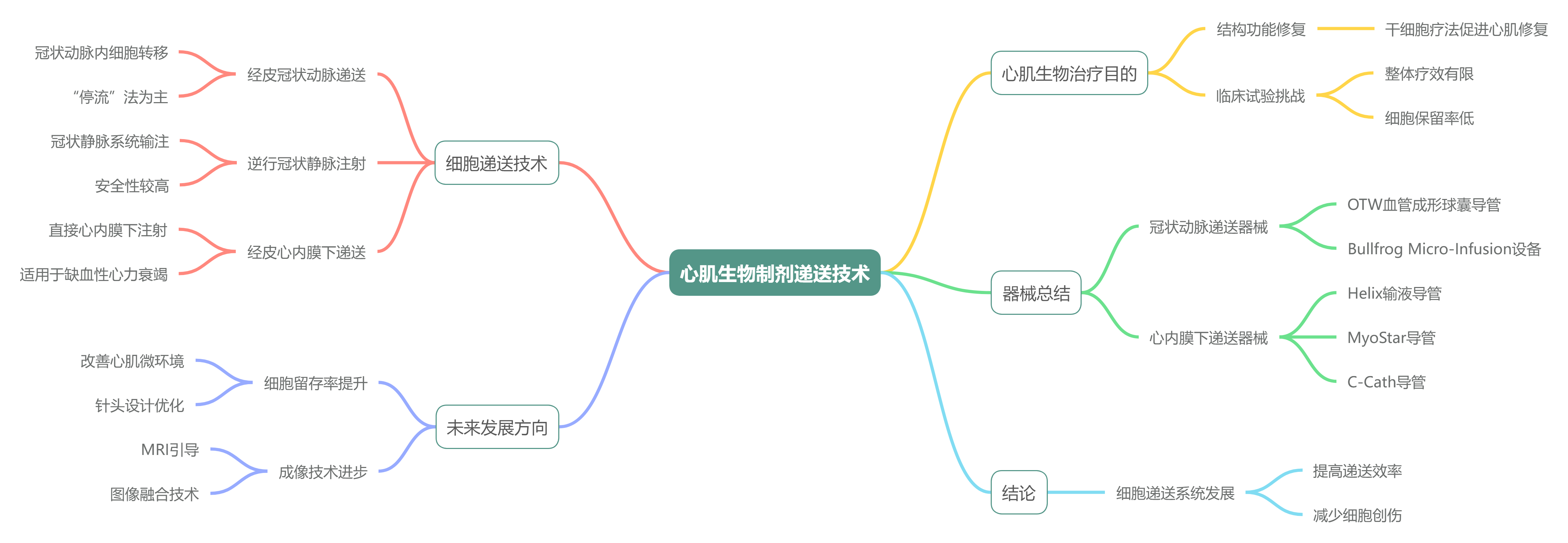
器械总结
以下是文中提到的所有器械的汇总表格:
| 器械名称 | 应用途径 | 特点 | 厂家名称 |
|---|---|---|---|
| Over-the-wire (OTW) 血管成形球囊导管 | 经皮冠状动脉细胞递送 | 用于非闭塞性或 “停流” 方法的细胞注射,类似于球囊血管成形术,进行全面抗凝、血流动力学和心电监测,配合 6 Fr or 5 Fr 导引导管 | 常规设备,未指定 |
| Bullfrog Micro-Infusion device™ Cricket™ | 冠状动脉外膜间隙递送 | 带球囊包覆的微针,可在血管外膜间隙递送生物制剂,提高细胞留存率,适用于大细胞,高安全性。 | Mercator Medsystems Inc, 美国加利福尼亚州圣雷昂多 |
| PiCSO™ 系统 | 逆行冠状静脉输注 | 包含控制台和一次性球囊导管,通过间歇性提升冠状窦压力促进内源性修复,适用于直接 PCI 期间。 | Miracor Medical, 比利时 |
| Helix™ 输液导管 | 经皮心肌内递送 | 带螺旋形 25 号针头,可扩展至 4 毫米,双腔注射,造影端口,旋转针头进行固定,适用于心内膜递送。 | BioCardia Inc., 美国加利福尼亚州旧金山 |
| Morph™ 导管 | 经皮心肌内递送 | 随 Helix™ 导管使用,用于引导至左心室,作为推进 Helix™ 导管的一部分。 | BioCardia Inc., 美国加利福尼亚州旧金山 |
| MyoStar™ 导管 | 经皮心肌内递送 | 集成 NOGA® XP 系统进行机电引导,带 27 号可调针头,基于诊断图进行数据驱动的定位。 | Biologics Delivery Systems, 美国加利福尼亚州钻石吧 |
| MyoCath™ | 经皮心肌内递送 | 与 MyoStar™ 类似,带手动可伸缩针头。 | Bioheart Inc. |
| NOGA® XP 导航系统 | 经皮心肌内递送 | 3D 机电图绘制系统,实时提供局部激活时间、单极电压、双极电压和线性局部缩短 LLS 数据,集成 MyoStar™ 和 MyoCath™。 | Cordis, Johnson & Johnson, 美国新泽西州沃伦 |
| C-Cath | 经皮心肌内递送 | 8Fr 可调弯头导管,含 28 号可回缩镍钛针,有 7 个侧孔,设计用于更好的细胞分散和保留。 | Celyad, 比利时蒙圣吉伯特 |
# Summary 概述
The primary objective of biologic therapy including stem cell based cardiovascular regenerative approach is to aid structural and functional repair of the myocardium. To date, overall clinical experience indicates that this therapy is feasible and safe. Structural and functional benefits remain overall neutral with signals of efficacy in well-defined patient cohorts.
生物治疗,包括基于干细胞的心血管再生方法,其主要目标是帮助心肌的结构和功能修复。到目前为止,总体临床经验表明,这种疗法是可行和安全的。结构和功能益处总体上保持中性,但在特定患者群体中显示出疗效迹象。
A reoccurring pitfall appears to be variable biodistribution and low rates of cell retention by the recipient myocardial tissue. Furthermore, clinical studies indicate a progressive decline in myocardial signals after delivery of labelled stem cells, a finding consistent with rapid cell death or washout within hours of administration.
一个反复出现的问题似乎是生物分布的变异性以及接受者心肌组织的细胞保留率低。此外,临床研究表明,在注射标记的干细胞后,心肌信号会逐渐下降,这一发现与细胞在给药后数小时内迅速死亡或被清除一致。
Ongoing debate exists as to the most appropriate technique or device for stem cell delivery. The selection depends on the assessment of the underlying pathology, the acuteness of myocardial injury or associated interventional procedure as well as the actual cell type to be delivered.
关于干细胞递送的最适当技术或设备的讨论仍在继续。选择取决于对潜在病理、心肌损伤的急性程度或相关介入手术以及实际要递送的细胞类型的评估。
Whilst in the setting of STEMI, intracoronary cell transfer is preferred, direct endomyocardial delivery is the method of choice as a stand-alone procedure in ischemic heart failure. This chapter describes in detail the respective strategies and devices used for cell delivery as well as novel approaches to improve cell retention.
在 STEMI 的背景下,冠状动脉内细胞转移是首选,而在缺血性心力衰竭中,直接心内膜下递送是作为独立手术的首选方法。本章详细描述了用于细胞传递的相应策略和设备,以及改善细胞保留的新方法。
# Introduction 介绍
Biologic -based cardiac regenerative therapies objective is to halt or even reverse the events responsible for the progression of heart failure (details see chapter Mozid et al, Cell based therapy). Clinical experience with cell therapy demonstrates that the approach is feasible and safe [1] , [2].
基于生物制剂的心脏再生疗法的目标是,阻止甚至逆转导致心力衰竭进展的事件(详情见 Mozid 等人的章节,基于细胞的治疗)。临床使用细胞治疗的经验表明,该方法是可行且安全的。
Though with sound conceptual evidence, overall structural and functional benefit was reported modest in magnitude across heterogeneous patient populations [1] , [2]. However, clinical trial experience has identified several factors which may influence the outcome of therapy [3] , [4].
尽管有坚实的理论证据,但在异质患者群体中,整体结构和功能的改善程度报告为中等。然而,临床试验经验已经确定了几个可能影响治疗结果的因素。
They include patient-specific variables such as functionality or priming of adult stem cells, extent of cardiac remodeling, or patient-independent parameters, such as stem cell processing [3] , [4] , [5] .
这些因素包括患者特异性变量 ,如成体干细胞的功能或活化程度,或心脏重塑的程度,以及患者独立参数,如干细胞处理。
A showcase of the co-ordinated effort to identify scalable and effective regenerative therapy is the clinical experience with cardiopoietic cell therapy in ischemic heart failure responsive patient population with optimal treatment intensity [5].
在缺血性心力衰竭患者群体中,造心细胞疗法以最佳的治疗强度获得了临床经验,这充分展示了为确定可扩展的有效再生疗法所做的协调努力。
New knowledge gained in that specific experience informs also the next generation regenerative therapeutics that can be manufactured as engineered cellular or secretome mimicking cell-free platforms. Launching biotherapeutics tailored for optimal outcome and offered at mass production cost would contribute in advancing equitable regenerative care that addresses population health needs [5].
在这一特定经验中获得的新知识也对下一代可制造的再生疗法有启示,这些疗法可以作为工程化的细胞或模拟分泌组的无细胞平台。以大规模生产成本推出专为最佳效果定制的生物治疗药物,将有助于推进公平的再生护理,满足人口健康需求。
Independently of the types of biologics, common limitations in cardiac regenative interventions include poor cell retention to the targeted destination to ensure optimal treatment intensity and the lack of consensus surrounding the optimal cell delivery methods [6] , [7] , [8].
不论生物制品的类型如何,心脏再生干预常见的局限性包括,细胞在靶向目标的滞留率低下不足以确保最佳治疗强度,以及缺乏最佳细胞递送方法的共识。
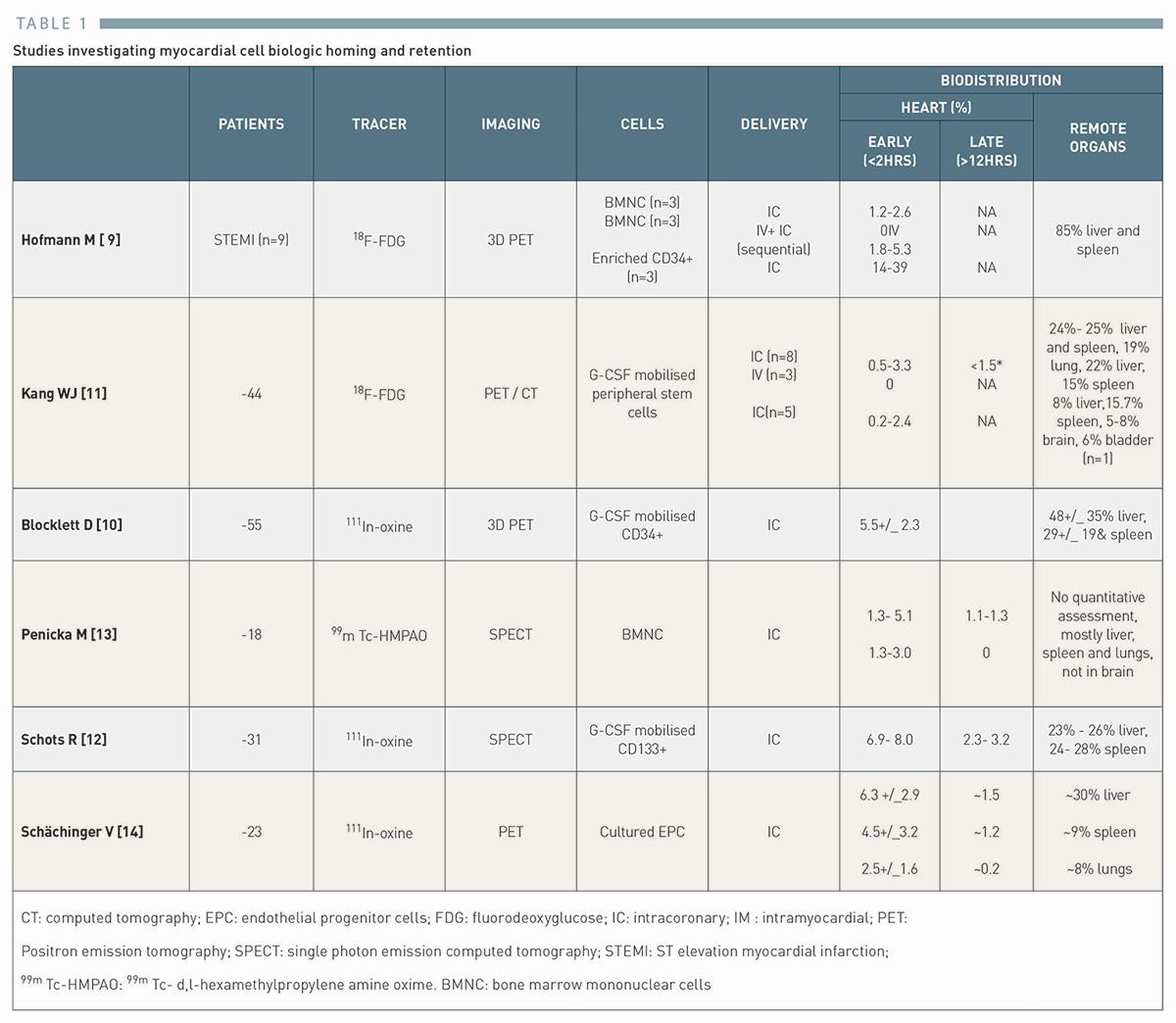
To date, retention of biologics has proved problematic with typically no greater then 5 to 10% of the injected dose remaining in targeted areas regardless of delivery of methods [9] , [10] , [11], [12] , [13] , [14](Table 1).
迄今为止,生物制剂的保留问题一直难以解决,无论采用何种递送方法,通常在目标区域仅能保留注射剂量的 5% 至 10%。
Variances in biodistribution exists, depending in part on the cell type [9], with a significant proportion of cells lodging in distant organs such as the lungs, liver or spleen.
生物分布存在差异,这在一定程度上取决于细胞类型,相当一部分细胞会滞留在远端器官,如肺、肝或脾。
The clinical significance of extracardiac seeding of biologic cells has not been established and bio-vigilance monitoring has been incorporated in the development of biologic based interventions as was previously carried out in traditional small molecule drug development.
生物细胞在心脏外播散的临床意义尚未确立,生物监测已被纳入生物干预措施的研发中,正如以往在传统小分子药物开发中所实施的那样。
Following biologic cell administration, studies highlight progressive loss of myocardial signals,[11] , [13] , [14] in keeping with poor cell delivery, rapid cell death or washout post often within hours of implantation.
生物细胞注射后,研究指出心肌信号逐渐减弱,这与细胞递送不良、植入后数小时内细胞迅速死亡或流失有关。
This limitation does not necessarily negate the efficacy of stem cells.
这一限制并不必然否定干细胞的效用。
Rather, it suggests that regenerative mechanisms involve a collusion of paracrine or immunomodulatory processes that may not require long term incarceration of intact cells.
相反,这表明再生机制涉及旁分泌或免疫调节过程的协同作用,这些过程可能不需要长期监禁完整的细胞。
The present chapter will review current techniques of cell delivery and outline the path for further development to improve cell retention and cell survival upon delivery.
本章将回顾当前的细胞递送技术,并概述进一步开发的途径,以改善细胞递送后的细胞存留和存活率。
In Table 2 there is a summary of the current techniques for cell delivery. Cell therapy can be delivered indirectly by peripheral injection, or by mobilization using cytokine therapy.
表 2 总结了当前细胞递送技术。细胞疗法可通过外周注射间接递送,或借助细胞因子疗法实现动员。
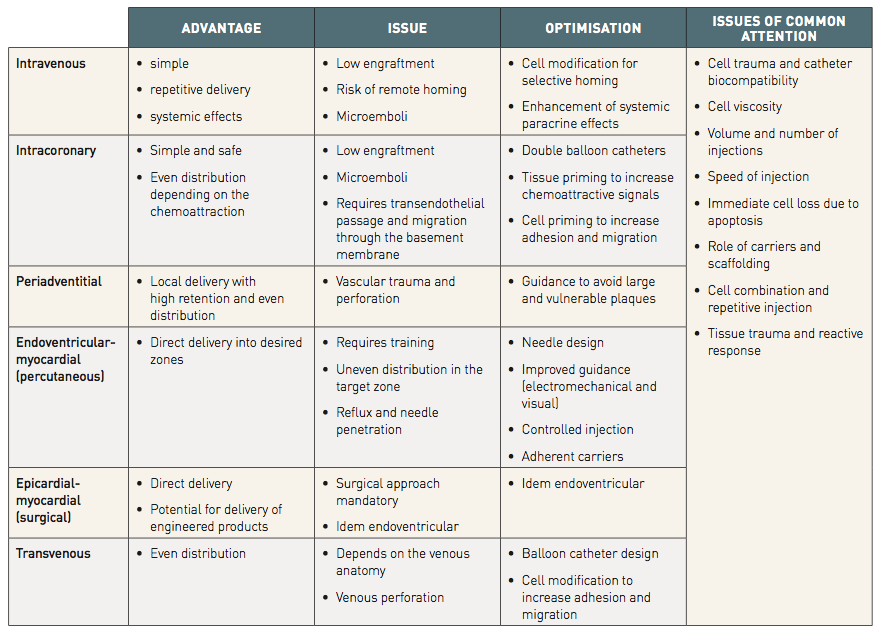
Delivery into the heart is also possible, either via the coronary arteries, coronary sinus or direct intramyocardial injection.
心脏内递送亦可实现,途径包括冠状动脉、冠状静脉窦或直接心肌内注射。
Here, we focus on the invasive percutaneous interventional techniques of coronary and direct myocardial cell delivery.
在此,我们着重探讨冠状动脉及直接心肌介入的经皮侵入性技术。
# FOCUS BOX 1
Cardiac stem cell therapy
- Cell and biologic therapies are an emerging therapy used as an adjunctive treatment after STEMI, chronic myocardial ischemia and in patients with ischemic or non-ischemic congestive heart failure
细胞和生物疗法是一种新兴疗法,可用于 STEMI、慢性心肌缺血以及缺血性或非缺血性充血性心力衰竭患者的辅助治疗。 - Factors affecting the final biological and functional therapeutic outcome are multiple and relate to patient-specific variables, such as functionality of autologous cells, choice of cell type or patient-independent parameters, such as stem cell processing
影响最终生物学和功能性治疗效果的因素是多方面的,与患者特异性变量相关,如自体细胞的功能性、细胞类型的选择,或与患者无关的参数,如干细胞处理。 - Low rates of cell retention and variable biodistribution are common features of current clinical experience which will require attention in the future clinical adoption of cellular strategies
细胞存留率低和生物分布不稳定是目前临床经验的共同特点,需要在未来临床采用细胞策略时加以注意。
# Percutaneous coronary cell delivery 经皮冠状动脉细胞递送
A coronary therapeutic approach is aimed at augmenting endogenous myocardial salvage by facilitating the trans endothelial implantation of biologic cells and their homing to the site of the injured myocardium. It is based on a combination of factors which increase myocardial cell retention after myocardial injury.
冠状动脉治疗途径,旨在通过促进生物细胞的跨内皮植入并归巢至受损心肌部位,增强内源性心肌挽救。这种方法基于多种因素的结合,这些因素可在心肌损伤后,增加心肌细胞的存留。
They include cell adhesion onto the endothelial layer, trans endothelial(vascular) passage and migration, and the presence of chemo attractive and adhesive forces in the myocardium, leading ultimately to cell retention in the perivascular region and within the myocardium itself.
它们包括细胞粘附到内皮层、跨内皮(血管)通过和迁移,以及心肌中存在的化学吸引力和粘附力,最终导致细胞滞留在血管周围区域和心肌内部。
This, one of the more technically feasible approaches, has been the method of choice for almost all studies in patients with STEMI. Percutaneous coronary delivery has also been the approach applied in a few studies in chronic myocardial ischemia or ischemic LV dysfunction.
这种方法由于其技术可行性较高,已成为几乎所有 STEMI 患者研究的首选手段。经皮冠状动脉递送技术也已在少数慢性心肌缺血或缺血性左心室功能障碍的研究中得到应用。
The success of coronary transfer requires that target tissue be subtended by an angiographically patent coronary artery or identifiable collateral vessel. The potential advantage of such coronary cell transfer is the homogenous distribution of the cells in the subtended target territory.
冠状动脉转移的成功,要求靶组织须由血管造影显示通畅的冠状动脉或可识别的侧支血管所供应。 这种冠状动脉细胞转移的潜在优势在于细胞在目标区域的均匀分布。
The optimal time window for the coronary cells transfer depends on the balance between multiple putative and detrimental factors governing the infarction healing process. The balance appears to be most favorable between day 3 and 7 post-infarction. [15]
冠状动脉细胞转移的最佳时间窗口取决于影响梗死愈合过程的多种假定因素和不利因素之间的平衡。梗塞后第 3 天和第 7 天之间的平衡似乎最为有利。
However, the kinetics and timing of coronary arterial cell delivery have received little attention in preclinical studies and the transition from isolated small animal studies to humans has been rapid. Consequently, much of what is known regarding cell retention after intracoronary administration has been learned from clinical studies.
然而,冠状动脉细胞递送的动力学和时机在临床前研究中鲜少受到关注,从孤立的小动物研究到人体的过渡又颇为迅速。因此,关于经冠状动脉注射后细胞留存情况的大部分已知信息,多来自临床研究。
These studies were associated with a positive functional outcome if cell injection was performed beyond day three. [1] , [2]
这些研究结果表明,如果超过第三天时进行细胞注射,则会产生积极的功能结果。
On the other hand, efficacy of coronary cell transfer in the later stages or in a chronic setting is still the matter of ongoing research and requires a better understanding of humorally mediated homing.
另一方面,冠状动脉细胞移植在晚期或慢性环境中的转移效能仍是当前研究的主题,尚需对体液介导的归巢机制有更深入的理解。
Coronary cell transfer may be associated with a risk of micro-emboli in the case of large cells or highly viscous suspensions.
如果细胞体积较大或悬浮液粘稠度较高,冠状动脉细胞转移可能存在微栓子风险。
# The over-the-wire (OTW) angioplasty balloon catheter
Guide catheters (6 Fr or 5 Fr) are preferred for selective cell infusion, given the calibre of their internal diameters (ID).
导引导管(6 Fr 或 5 Fr)的内径(ID)口径较大,因此是选择性细胞输注的首选。
The technique typically utilizes off-the-shelf devices such as over-the-wire (OTW) angioplasty balloon catheters.
该技术通常采用现成的器械,如经导丝(OTW)血管成形球囊导管。
The basic set-up is similar to balloon angioplasty with full anticoagulation as well as haemodynamic and ECG monitoring.
基本设置与球囊血管成形术类似,都要进行全面抗凝以及血流动力学和心电图监测。
The most commonly applied technique of coronary cell transfer is anterograde delivery using selective coronary injections.
冠状细胞移植最常用的技术是采用选择性冠状动脉注射的顺行递送法。
Cells are injected through the central lumen of the delivery catheter whilst either maintaining coronary flow (non-occlusive) or interrupting it with balloon occlusion (“stop-flow” method).
细胞通过递送导管的中心管腔注入,同时保持冠状动脉血流(非闭塞)或用球囊闭塞中断血流("止流" 法)。
In both cases, care is taken so that the delivery catheter is flushed with saline prior to its use in order to prevent intraluminal thrombus formation.
在这两种情况下,均采取措施以确保递送导管在使用前用生理盐水冲洗,从而预防管腔内血栓形成。
In the case of the non-occlusive method, an angioplasty balloon catheter (or a specialty catheter) is used for sub-selective injection into the culprit vessel.
在非闭塞方法中,使用血管成形术球囊导管(或专用导管)对病变血管进行部分选择性注射。
The position of the catheter is determined by the extent of the target territory. The internal lumen is connected at its proximal end to the syringe containing the cell product. The cell product is then introduced either as a continuous infusion or by repeated slow hand injections.
导管位置由目标区域范围决定,其内腔近端与含细胞产品的注射器相连。然后以持续输注或反复缓慢手动注射的方式输入细胞产品。
In the case of hand injection, the cell product is loaded securely via a Luer lock using a small volume syringe, which thus allows for smooth and controlled injections.
在手动注射的情况下,使用小容量注射器,通过鲁尔锁安全地装入细胞产品,从而实现平稳、可控的注射。
Previous animal work suggests that continuous infusion may be more effective. [16]
先前的动物实验表明,持续输注可能更为有效。
However, “stop-flow” is the predominant method in clinical trials. This method is preferred especially after myocardial infarction (Figure 1).
不过,在临床试验中,"停流" 是最主要的方法。尤其是在心肌梗死后,这种方法更受青睐(图 1)。
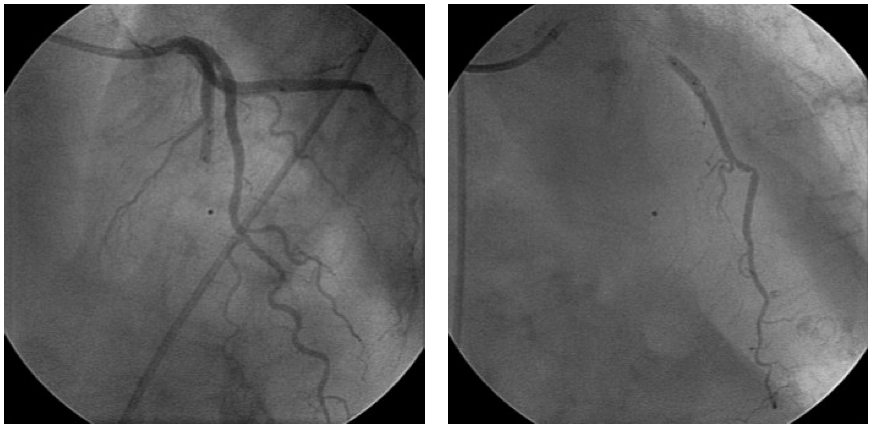
Figure 1. Intracoronary cell delivery using the “stop-flow” technique.
图 1. 利用 “停流” 技术进行冠状动脉内细胞递送。
The left panel demonstrates balloon occlusion at the site of the stented segment.
左图,显示支架段部位球囊阻塞情况。
Contrast injection confirms stop of the flow for the given balloon size at low pressure inflation.
造影剂注射确认,在低压充盈下,给定尺寸的球囊成功阻断血流。
Contrast injection through the internal balloon lumen right panel shows the expected cell distribution when injected during the balloon occlusion.
右图,通过球囊内腔注入造影剂,显示在球囊阻塞期间注入时的预期细胞分布。
A balloon angioplasty OTW catheter is placed at the site of the stented segment at the location of the culprit lesion of the infarct-related coronary artery.
将一个球囊血管成形术 OTW 导管置于支架段所在位置,即梗死相关冠状动脉的罪犯病变处。
Intracoronary nitrates should be administered prior to wire and balloon placement.
在放置导丝和球囊前,应给予冠状动脉内硝酸甘油。
Care should be taken to avoid an additional injury of the stented segment or surrounding vessel wall.
应谨慎操作,避免支架段或周围血管壁遭受额外损伤。
Accordingly, balloon length should be smaller than the nominal length of the stent. The size should be comparable or it may be at most 0.5 mm larger than the nominal size of the implanted stent.
因此,球囊长度应小于支架的标称长度。球囊的尺寸应与植入支架的标称尺寸相当,或最多比标称尺寸大 0.5 毫米。
Likewise, low pressure balloon inflations are applied that typically do not exceed the nominal inflation pressure.
同样,采用低压气球充气时,充气压力通常不会超过标称充气压力。
Before cell injection, test inflation followed by test contrast can be performed to document the coronary artery occlusion at the desired segment.
细胞注射前,可以先进行测试充气,然后再进行试验性造影,以记录所需节段的冠状动脉闭塞情况。
Cell product can be loaded via 3-way stop-cock connected to both to a small volume Luer lock syringe (3 ml) and a larger Luer lock syringe (10 or 20 ml).The system is then attached to the proximal internal lumen of the OTW balloon catheter.
细胞制品可通过三通止血阀进行加载,该止血阀连接至一个小容量鲁尔锁注射器(3 毫升)及一个大容量鲁尔锁注射器(10 或 20 毫升)。然后将该系统连接到 OTW 球囊导管的近端内腔。
After balloon inflation cells are injected typically in boluses of ~ 3 ml over ~ 1 minute period with a total occlusive time of 3 minutes.
球囊充气后,通常在 1 分钟内注入约 3 毫升的细胞,总闭塞时间为 3 分钟。
This timing is empirically chosen and is presumed to prolong the contact between infused cells and subtended endothelial surface of the coronary macro and microcirculation. Following a 3 minute period, reperfusion is allowed for 2 to 3 minutes.
这个时间是根据经验选择的,目的是延长注入细胞与冠状动脉大循环和微循环的内皮表面之间的接触时间。3 分钟后,再灌注时间为 2 至 3 分钟。
The “stop-flow” cycle is repeated until all the desired cell product is transferred.
“停流” 循环将持续重复,直至所有所需细胞产品完成转移。
During balloon occlusion, the patient is monitored for signs of ischemia, arrhythmias or haemodynamic instability.
在球囊阻断期间,需监测患者是否出现缺血、心律失常或血流动力学不稳定的迹象。
If severe arrhythmias, severe symptomatic ischemia or major haemodynamic instability are observed during balloon inflation, the procedure should be interrupted and injections modified according to patient tolerance.
如果在球囊充气过程中观察到严重的心律失常、严重的症状性缺血或严重的血流动力学不稳定,则应中断手术,并根据患者的耐受性调整注射量。
At the end of the procedure, vessel patency and flow in the coronary artery are checked angiographically.
手术结束时,应通过血管造影检查冠状动脉的血管通畅性和血流情况。
# The Bullfrog Micro-Infusion device
Addressing the challenge of low retention rates, a coronary cell delivery method using the periadventitial space as target area was developed using a dedicated micro-infusion catheter (Bullfrog Micro-Infusion device™ Cricket™; Mercator Medsystems Inc, San Leando, CA, USA).
针对保留率低的问题,研发了一种以冠状动脉外膜间隙为目标区域的细胞递送方法,采用专用微灌注导管(Bullfrog Micro-Infusion 设备™ Cricket™;Mercator Medsystems Inc, San Leando, CA, USA)进行操作。
This is task specific catheter, tipped with a balloon-sheathed microneedle, and is guided and inflated in a manner similar to an angioplasty catheter, but with far lower expansion pressures.
此为专用导管,头端配有球囊包裹的微针,其引导和充气方式与血管成形术导管相似,但膨胀压力要低得多。
The closed balloon provides a protective covering for a tiny injection needle as it is guided safely through the vasculature. When the desired injection site is reached, the balloon is inflated with saline, securing the system for injection and sliding the microneedle through the vessel wall.
闭合的球囊为微型注射针提供保护,引导其安全通过血管。当到达目标注射部位时,盐水充盈球囊,固定注射系统并使微针穿透血管壁。
This catheter has been used in at least one US-based clinical study on the injection of allogeneic biologics in patients with recent myocardial infarction.
这种导管至少在一项美国临床研究中使用过,该研究旨在为近期心肌梗死患者注射异体生物制剂。
The potential advantage of the catheter is a high retention rate after delivering the biologics to the periadventitial space, which serves as a reservoir for the target area.
这种导管的潜在优势在于,将生物制剂递送到血管外膜周围腔后,其保留率较高,该空间可作为目标区域的储液库。
A further advantage is that by avoiding coronary transfer it may be suitable for delivering biologics comprised of large cells.
另一个优点是,通过避免冠状动脉转移,它可能适合递送由大细胞组成的生物制剂。
While initially a concern existed regarding potential vessel damage in the presence of atherosclerotic lesions, fears were allayed, with a Phase I Clinical Study.
尽管起初担忧动脉粥样硬化斑块的存在可能造成血管损伤,但通过一期临床研究,这些顾虑得以消除。
The delivery of bone marrow derived adherent adult stem cell product using a first in coronary adventitial delivery system did not reveal major clinical safety issues. [17]
使用冠状动脉外膜递送系统首次递送骨髓来源的贴壁成体干细胞产品,未发现重大临床安全问题。
# Retrograde Coronary Venous Injection 逆行冠状静脉注射
In addition to antegrade coronary cell transfer, retrograde infusion of stem cells through the coronary sinus and coronary venous system has been achieved in experimental models [18].
除了顺行冠状动脉细胞移植外,在实验模型中已经实现了通过冠状窦和冠状静脉系统逆行输注干细胞。
Theoretically this approach should also be safer than intracoronary or intramyocardial injection. Initial case reports demonstrated feasibility of this technique [19].
理论上,这种方法应该比冠状动脉内或心肌内注射更安全。初步病例报告显示了该技术的可行性。
While robust trial evidence is sparse on this delivery technique, a small clinical trial has shown this to be a relatively safe route [20].
虽然关于这种给药技术的可靠试验证据并不多,但一项小型临床试验表明,这是一种相对安全的途径。
In this procedure, the coronary sinus is cannulated using a guidewire and an angioplasty balloon is advanced to the selected cardiac vein related to the coronary artery territory to be treated.
在此手术中,使用导丝对冠状窦进行插管,然后将血管成形球囊推进到与要治疗的冠状动脉区域相关的选择性冠状静脉。
Venous blood flow is then stopped, by prolonged balloon inflation for approximately 15 minutes, while the stem cells are infused under pressure.
然后,通过延长球囊充气时间约 15 分钟,来阻止静脉血流,同时在压力下注入干细胞。
The main limitations to this approach concern limitations in cell retention relative to the anterograde approach [20] whilst also being a technically challenging route owing to the more variable coronary venous anatomy (as compared to the coronary arterial tree) which can at times be difficult to negotiate with guidewires and balloons.
这种方法的主要局限性在于,与前向方法相比,细胞保留能力有限,而且由于冠状静脉解剖结构(与冠状动脉树相比)更加多变,导丝和球囊有时难以操作,因此在技术上也是一种具有挑战性的途径。
This technique may be further limiting in patients who already have a coronary sinus device in situ, such as a LV lead for resynchronization therapy or a coronary sinus annuloplasty device, although cells could be administered as an adjunct to the implantation of such devices.
对于已经植入了冠状窦装置(如用于再同步化治疗的左心室导联,或冠状窦瓣环成环装置)的患者来说,这种技术可能会受到进一步限制,尽管可以将细胞作为植入此类装置的辅助手段进行给药。
# PiCSO™
Pressure-controlled intermittent coronary sinus occlusion (PiCSO™, Miracor Medical, Belgium) is a novel device-based technology to stimulate endogenous repair by increasing intermittently coronary sinus pressure and coronary sinus pulse pressure.
压力控制间歇性冠状窦闭塞术(PiCSO™, Miracor Medical, 比利时)是一项新型器械辅助技术,通过间歇性提升冠状窦压力及冠状窦脉压,刺激内源性修复。
The system consists of a console which is controlled by a graphical user interface and a single-use balloon catheter.
该系统包括一个由图形用户界面控制的控制台和一个一次性球囊导管。
The catheter contains four lumens, a balloon on the distal end, and soft tip and connectors on the proximal end for the shuttle gas supply and coronary sinus pressure measurement.
导管有四个管腔,远端有一个球囊,近端有软头和连接器,用于供应穿梭气体和测量冠状窦压力。
The system saves a log file for each patient from which the device performance can be determined, i.e. increase in coronary sinus pressure during inflation cycles. Therapy is applied in the setting of the primary PCI.
系统为每位患者保存日志文件,据此可评估设备性能,例如充气周期中冠状窦压力的增加情况。在直接经皮冠状动脉介入治疗(PCI)过程中实施该疗法。
The proposed mechanism of action is postulated to a reduction of ischemic injury by re-distributing blood flow and improving microvascular function facilitating infarct size reduction. Consistent with the Initial clinical studies demonstrate benefits on infarct size as adjunct to primary PCI in the STEMI setting [21].
据推测,其作用机制是通过重新分配血流和改善微血管功能来减少缺血性损伤,从而促进梗死面积的缩小。初步临床研究表明,在 STEMI 情况下,作为直接 PCI 的辅助手段,有助于减小梗死面积。
# FOCUS BOX 2
Coronary cell delivery 冠状动脉细胞递送
- Coronary cell transfer using the “stop-flow” method is the preferred method of delivery early after STEMI
使用 "停流" 法进行冠状动脉细胞转移是 STEMI 后早期递送细胞的首选方法 - The optimal time window for coronary delivery depends on the balance between multiple putative and detrimental factors governing the infarction healing which appears to be most favorable between day 3 and 7 after the infarction
冠状动脉递送的最佳时间窗取决于多个潜在益处与不利因素之间的平衡,这些因素影响梗死愈合的过程,研究表明最适宜的时间段为梗死后第 3 至第 7 天。 - Over-the-wire balloon catheter are the most widely used device for cell transfer
导丝球囊导管是细胞转移最广泛使用的装置 - New devices such as an infusion catheter allowing the periadventitial cell delivery may offer higher retention rates. Early experience suggest a favorable safety profile of the device
新型装置如输注导管,允许血管外膜细胞递送,可能提供更高的保留率。早期经验表明该装置具有良好的安全性。 - The clinical efficacy of retrograde coronary venous infusion remains limited by concerns with biologic cell retention . Novel device-based control of coronary sinus pressure may offer a new approach improving microvascular function as adjunct to primary PCI
逆行冠状静脉输注的临床疗效仍然受到生物细胞滞留问题的限制。基于新型设备的冠状动脉窦压力控制可提供一种改善微血管功能的新方法,作为直接 PCI 的辅助手段
# Percutaneous intramyocardial cell delivery 经皮心肌内细胞递送
Direct myocardial (intramyocardial) injection is the preferred choice of cell delivery in the setting of ischemic heart failure or chronic myocardial ischemia.
直接心肌(心肌内)注射是缺血性心力衰竭或慢性心肌缺血情况下细胞递送的首选方法。
It can be performed trans-epicardially, typically as adjunct to coronary bypass surgery, or percutaneously with endoventricular injection as a standalone procedure [22].
可以经心外膜进行(通常作为冠状动脉搭桥手术的辅助手段),也可以经皮进行心内膜注射,作为一种独立的手术。
Percutaneous 8Fr femoral arterial access with retrograde left ventricular approach (with the catheter passing the aortic valve into the left ventricle) is the preferred choice of direct myocardial delivery.
经皮 8Fr 股动脉穿刺逆行左心室途径(导管经主动脉瓣进入左心室)是直接心肌内给药的首选方法。
This approach can also be applied safely to high risk patients without the need for general anesthesia or sternotomy by targeting the injections into the desired myocardial areas and, in principle, may also allow a repeat injection as part of a serial treatment strategy.
这种方法还可以安全地应用于高风险患者,无需全身麻醉或胸骨切开术,只需将注射目标锁定在所需的心肌区域,原则上还可以重复注射,作为连续治疗策略的一部分。
Initial operator inexperience has been associated with purported lack of uniformly distributed cell injections and is associated with, an albeit minimal, risk of perforation or tissue injury.
最初术者由于缺乏经验,导致细胞注射分布不均匀,并有可能造成穿孔或组织损伤,尽管这种风险很小。
In addition, the percutaneous myocardial delivery requires either fluoroscopic or dedicated three-dimensional imaging guidance.
此外,经皮心肌注射需要透视或专门的三维成像引导。
Historically, several trans-endocardial injection catheters have been developed. The most frequently used catheters in clinical trials (Table 3) include :
历史上曾开发过多种经心内膜注射导管。临床试验中最常用的导管(表 3)包括:
the Helix™ infusion catheter (BioCardia Inc., San Francisco, CA, USA), MyoStar™ (Biologics Delivery Systems, Diamond Bar, CA, USA) and C-Cath (Celyad, Belgium).
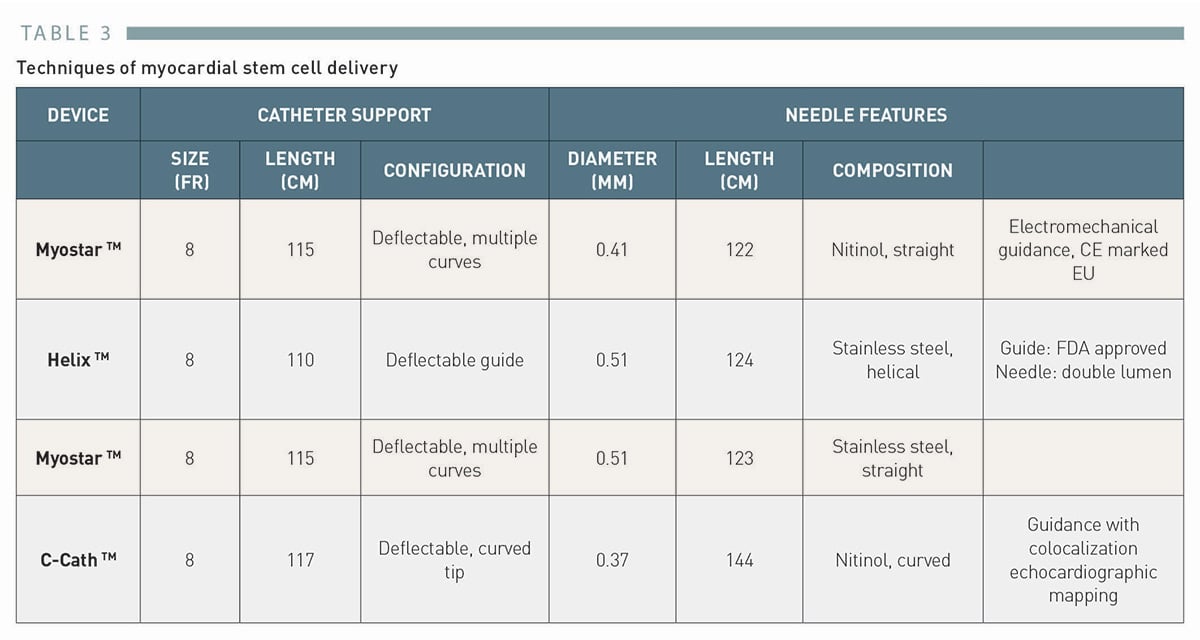
# The Helix™ catheter
BioCardia’s Helix bio-therapeutic delivery catheter system (Figure 2) consists of a helical 25 gauge needle, expandable up to 4 mm.
BioCardia 的 Helix 生物治疗递送导管系统(图 2)包含一个螺旋形 25 号针头,最大可扩展至 4 毫米。
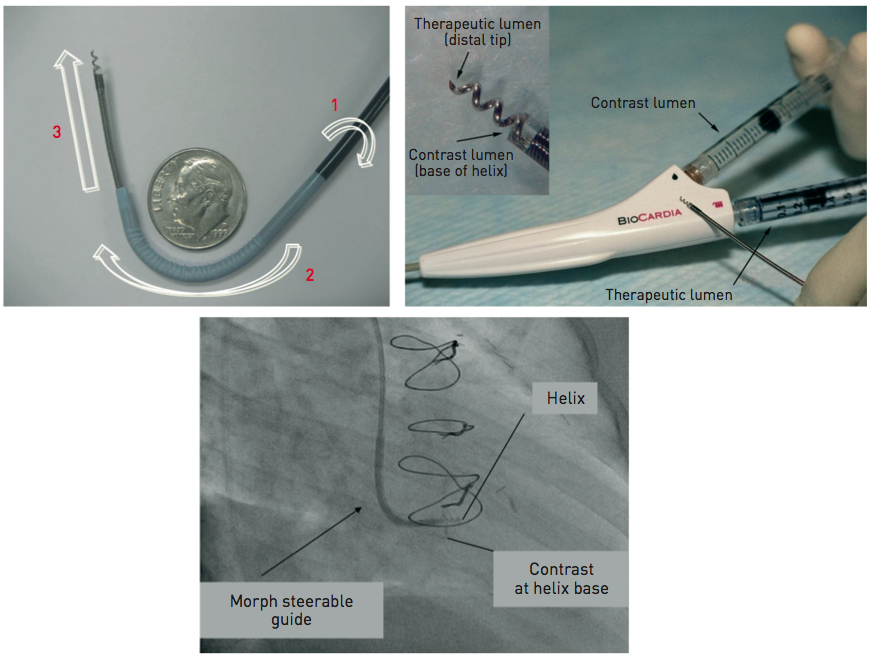
Figure 2. The Helix™ catheter for endomyocardial delivery
It consists of a double lumen injection catheter with a helical-shaped needle and an independent deflectable guide (upper left panel).
它由带有螺旋形针头的双腔注射导管、和独立的可调弯导管组成(左上图)。
The needle has a contrast port to confirm the endomyocardial position and an injection port for the cell delivery (upper right panel).
针头有一个用于确认心内膜位置的造影端口,还有一个用于细胞递送的注射端口(右上图)。
The lower panel shows a fluoroscopic image confirming the endomyocardial position at the time of the cell injection.
下图是细胞注射时确认心内膜位置的透视图像。
(Image courtesy of BioCardia, San Francisco, CA, USA)
Under fluoroscopic guidance, the system is advanced to the left ventricle over the guidewire with the Morph™ guide catheter (BioCardia Inc.).
在透视引导下,系统通过导丝及 Morph™导管(BioCardia 公司)被推进至左心室。
The deflectable system allows three degrees of freedom to facilitate operator control.
可调弯系统允许三个维度自由操控,以方便术者控制。
The helical, corkscrew needle is rotated into the heart tissue to provide active fixation during therapeutic delivery, similar to the active fixation electrodes used in cardiac pacing.
螺旋状针头旋转进入心脏组织,在治疗过程中提供主动固定,类似于心脏起搏中使用的主动固定电极。
The position and fixation of the needle is checked by injecting small bolus of a contrast through the second barrel contrast port located at the base of the helical needle.
通过向位于螺旋针底部的第二个造影端口,注射少量造影剂团块,检查针的位置和固定情况。
# The MyoStar™ catheter and the NOGA® XP navigational system
The MyoStar™ catheter is integrated with the NOGA® XP navigational system of electromechanical guidance (Cordis, Johnson & Johnson, Warren, NJ, USA). The system is not presently marketed.
MyoStar™导管与 NOGA® XP 导航系统集成。该系统目前尚未上市。
Given that it has been most frequently used device for cell delivery in the clinical trials and prototype of targe delivery, its conceptual description is of educational value for interventionalist. Cell delivery has been performed after obtaining a diagnostic endocardial map of the left ventricle.
鉴于它是临床试验中最常用的细胞递送装置和靶标递送原型,其概念描述对介入医生具有教育价值。在获得左心室的诊断心内膜图后进行细胞递送。
The NOGA® XP System produces a 3D electromechanical map by analysing parameters generated from intracardiac electrograms acquired at multiple endocardial locations which characterise mechanical, dynamic and electrical functions of the mapped cardiac chamber (Figure 3 and Figure 4).
NOGA® XP 系统通过分析从多个心内膜位置采集的腔内心电图生成的参数,绘制出三维机电图,这些参数描述了所绘制心腔的机械(如收缩和舒张)、动态(如血流变化)和电气(如电活动)功能(图 3 和图 4)。
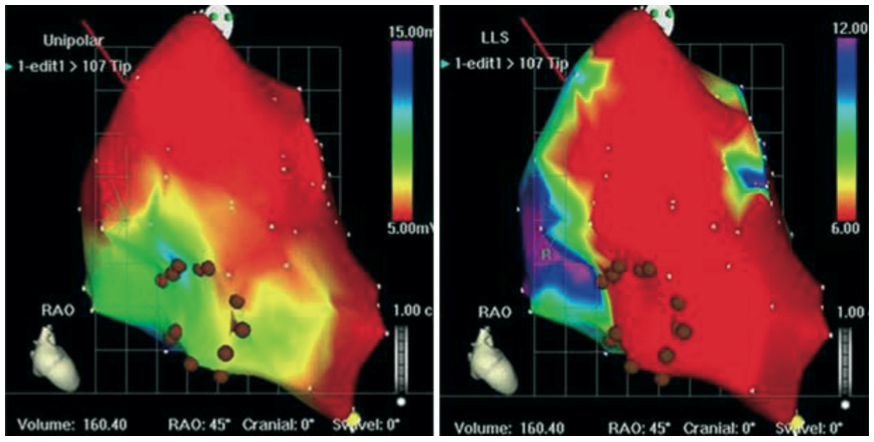
Figure 3. Left ventricular electromechanical map obtained by NOGA® XP system
NOGA® XP 系统获得的左心室机电图
The left panel shows the unipolar voltage map in a patient with ischaemic cardiomyopathy with threshold set at 5 mV.
左图显示了缺血性心肌病患者的单极电压图,阈值设置为 5 mV。(表明在该电压以上的区域被视为有较强的电生理活动,这有助于识别心肌的健康状态。)
The right panel shows linear local shorteningLLSat corresponding points of measurement.
右图显示了相应测量点的线性局部缩短LLS。(反映了心肌收缩的有效性,LLS 减少可能提示心肌功能障碍。)
Brown dots indicate injection sites in the areas of viable but dysfunctional myocardium, i.e., areas with preserved electrical activity with unipolar voltage above 5 mV and reduced LLS (Moving image 1).
棕色点表示在可存活但功能不全的心肌区域的注射位置,即电活动得以保留的区域,其单极电压超过 5 mV,但 LLS 减少(动态图像 1)。
Both diagnostic (NOGASTAR®; Cordis, Johnson & Johnson) and injection catheters can be manipulated with six degrees of freedom (x, y, z, roll, pitch and yaw) within the left ventricle.
诊断导管(NOGASTAR®)与注射导管均可在左心室内进行六个纬度的自由操作(x 轴、y 轴、z 轴,以及滚转、俯仰和偏航)。
"六个自由度" 指的是在三维空间中物体的运动方式:
x、y、z 轴:表示在空间中的平移运动,分别对应于左右、前后和上下的移动。
滚转(roll):指导管围绕其长轴的旋转。
俯仰(pitch):指导管围绕横轴的旋转,表现为向上或向下的倾斜。
偏航(yaw):指导管围绕垂直轴的旋转,表现为左右的转动。
In the first step, data are gathered from the location sensor and the electrical signal from the tip electrode of the diagnostic catheter.
第一步,通过位置传感器和诊断导管头端电极的电信号收集数据。
The system then measures the position and distance of each point in relation to the other points, as well as local electrical activity and mechanical movement associated with each point. The derived data generated can determine the end-diastolic and end-systolic volumes and ejection fraction.
然后,系统会测量每个点相对于其他点的位置和距离,以及与每个点相关的局部电活动和机械运动。生成的衍生数据可确定舒张末期和收缩末期容积以及射血分数。
In real-time, each acquired point provides data on the local activation time, unipolar and bipolar voltage amplitudes.
每个采集点都能实时提供局部激活时间、单极性和双极性电压振幅的数据。机械信息是根据索
Mechanical information is computed from the average change in distance between the index catheter point and points from end-diastole to end-systole, and presented as a linear local shortening LLS .
引导管点与舒张末期至收缩末期各点之间距离的平均变化计算得出的,并以线性局部缩短 LLS 的形式呈现。
The map which is then constructed provides views from multiple positions, including a bull’s-eye segmental view used to review the even distribution of regional points.
随后绘制的地图可提供多个位置的视图,包括用于查看区域点均匀分布的靶心分段视图。
Volumetric and electromechanical data correlate with other imaging modalities such as perfusion or MRI studies. After a diagnostic map has been constructed, the target area is delineated based on the combined information from unipolar voltage and LLS data.
容积和机电数据与灌注或核磁共振成像研究等其他成像模式相关。构建诊断图后,根据单极电压和 LLS 数据的综合信息划定靶区。
Normal myocardium exhibits unipolar voltages greater than 10 to 12 mV and LLS above 9% to 10%. Akinetic regions with LLS < 2% to 3% and unipolar voltage lower than 4 mV are considered to represent the infarct scar.
正常心肌的单极电压高于 10 至 12 mV,LLS 高于 9% 至 10%。LLS 为 2% 至 3%、单极电压低于 4 mV 的非运动区域,被认为是梗死瘢痕区。
Generally, areas of reduced LLS with unipolar voltage in the range of 6 to 9 mV represent viable myocardium being the target of therapeutic intervention.
一般来说,单极电压在 6 至 9 mV 范围内的 LLS 降低区域代表有活力的心肌,是治疗干预的目标。
Endo-ventricular injections are then performed with the MyoStar™ catheter, which retains the identical diagnostic properties, but it is also equipped with a 27 gauge adjustable needle.
随后通过 MyoStar™导管进行心室内注射,该导管保留了相同的诊断性能,同时配备了 27 号可调针头。
Injections must be performed by a dedicated team, preferably with 2 operators and a technician behind the NOGA workstation.
注射须由专门的团队执行,理想情况下应包括两名术者和一名位于 NOGA 工作站后的技术员。
The needle length is adjusted on a proximal handle consisting of an adjustable injector thumb knob, a hub for connection with the injection syringe.
针头长度可通过近端手柄调节,该手柄包含一个可调式注射器拇指旋钮及用于连接注射器的主轴。
During injections the quality criteria are set to ensure the optimal biologics delivery and retention. They include the catheter stability, confirmation of the unipolar voltage in the desired range. Each injection site is marked on the controlling map to ensure the even spread of the injections to the target area.
在注射过程中,要设定质量标准,以确保最佳的生物制剂递送和保留。这些标准包括导管的稳定性、单极电压在所需范围内的确认。每个注射点都标注在控制图上,以确保注射均匀扩散到目标区域。
Figure 4. Endoventricular delivery with Myostar™ catheter
The upper left panel shows the catheter handling with 2 operators, one controlling the catheter position and the second performing the cell injection.
左上图显示的是导管操作,有两名术者,一名控制导管位置,另一名进行细胞注射。
The upper right panel shows the needle in the injection position: note the curved shape of the catheter with a needle penetrating the endomyocardium in the perpendicular position.
右上图显示注射位置的针头:注意导管的弯曲形状,针头在垂直位置穿透心内膜。
The lower panel highlights the key quality criteria set to ensure the optimal biologics delivery with the system (Moving image 2).
下图强调了为确保使用该系统提供最佳生物制剂而设定的关键质量标准(动态图像 2)。
Injection catheter perpendicular to the wall 注射导管垂直于心室壁
Ventricular ectopy at needle protrusion 针头突出时出现心室异常搏动
Reliable catheter stability, LS < 3mm 可靠的导管稳定性,位移小于 3 毫米
Though the navigation is designed to be non-fluoroscopic, x-ray guidance typically facilitates the inter-region manipulation of the catheter.
虽然导航设计为非荧光透视,但 X 线引导通常有助于在区域间操作导管。
To ensure accuracy, it is critical to avoid any positional shift of the heart during the procedure. Accordingly, deep sedation or even general anaesthesia is required.
为确保准确性,手术过程中避免心脏位置移动至关重要。因此,需要进行深度镇静甚至全身麻醉。
Navigation and accuracy can also be compromised by catheter-induced bundle branch block or variable cycle length.
导管引起的束支阻滞或心动周期的变化也会影响导航和准确性。
The diagnostic performance as well as the interventional guidance to establish electrical and mechanical thresholds can be difficult in large ventricles with severely depressed global function. In such cases, unipolar voltage thresholds to delineate viable areas may need to be reduced, often to 4 to 5 mV.
对于整体功能严重减退的大心室来说,诊断和介入指导以建立电阈值和机械阈值都很困难。在这些情况下,可能需要降低用于界定存活区的单极电压阈值,通常降至 4 到 5 mV。
Other limitations are the time needed to construct the diagnostic map prior to the injection and the use of 2 catheters for completing the procedure, driving up the cost of the cell therapy.
其他限制包括在注射前构建诊断图所需的时间,以及使用两根导管完成手术,从而增加了细胞疗法的成本。
# The C-Cathez catheter
C-Cathez (Celyad, Mont-Saint Guibert, Belgium) has been developed on detailed fluid-dynamic modeling, a curved nitinol needle containing side-holes was incorporated into a deflectable tip delivery catheter.
C-Cathez 是根据详细的流体动力学模型开发的,它将含有侧孔的弧形镍钛合金针头与可调弯递送导管结合在一起。
C-Cathez is an 8Fr deflectable tip catheter containing a unibody 28 gauge retractable Nitinol alloy needle, featuring a curved tip with 7 side holes (Figure 5) [23].
C-Cathez 是一种 8Fr 的可调弯导管,内置一体式 28 号可回缩的镍钛合金针,其特点在于针头弯曲和有 7 个侧孔(图 5)。
Panel A: C-Cathez®
Panel B: Example of tissue distribution of radiocontrast injection in normal porcine myocardium. Right panel showing elliptical-shaped blush extending from catheter tip (needle not visible).
正常猪心肌内造影剂注射的组织分布示例。右侧图像显示从导管头端延伸出的椭圆形染色区域(针头不可见)。
Panel C: Co-localization schema target region identification for biologic injections using C-Cath.
图 C:利用 C-Cath 进行生物注射的共定位模式目标区域识别。
Example from the CHART-1 trial CHART-1 试验示例
Lower left panel C: “Bull’s eye” display, on a circumferential polar plot, of the 17 LV myocardial segments utilized for determination of injection targets. Segments shaded in red denote “no-go” zones and in orange denote regions to “approach with caution”.
左下角图 C:在环状极坐标图上展示用于确定注射目标的 17 个左室心肌节段的 "靶心"。红色阴影区表示 “禁入区”,橙色阴影区表示 “需谨慎接近区”。
Lower right panel C: Images from biplane left ventriculography with corresponding segments #’s from Bull’s eye map, marked. Cardiac landmarks include RV ICD lead and epicardial lead over mid anterolateral segment (#12 on ASE echo-map). Bidirectional arrow in small in upper-center inserts denotes diameter of pigtail catheter in each view (approximately 1.5 cm for this patient).
右下图像 C:双平面左心室造影图像,已标记对应于靶心图上的节段编号。心脏标志物包括右心室 ICD 导线和位于前外侧中段(ASE 心超图上的第 12 号区域)的心外膜导线。上方小插图中的双向箭头表示各体位下猪尾导管的直径(此患者约为 1.5 厘米)。
The handle contains manual controls for adjusting distal catheter curvature, for advancing and retracting the needle, and for setting the needle’s extended length.
手柄内含手动控制装置,用于调节导管远端的弯度,以及推进和回缩针头,还可设定针头的延伸长度。
A separate luer-lock portal for needle irrigation and injection arises from the handle’s back end. The proximal handle contains controls for needle deployment (“pusher”), for length adjustment (threaded mechanism distal to the Luer Lock) and needle retraction. The needle can be advanced beyond the catheter tip to predetermined lengths (3-6 mm).
手柄后端有一个独立的鲁尔锁接口,用于针头灌洗和注射。近端手柄包含用于针头展开("推杆")、长度调节(鲁尔锁远端螺纹装置)和针头回缩的控制装置。针头可推进到导管头端外的预定长度(3-6 毫米)。
Pre-procedural planning is similar to that of other elective interventional procedures and include echocardiography to define regions of the LV suitable for injection.
手术前的规划与其他择期介入手术相似,包括利用超声心动图确定适合注射的左心室区域。
Irrespective of wall thickness, apical, basal inferior and basal inferolateral, are considered ineligible for injection.
无论室壁厚度如何,心尖部、基底下部和基底下外侧,均不符合注射条件。
Other segments with wall thickness > 8 mm are eligible for injection with cautions in the areas of the conduction systems in the basal antero and infero-septal areas.
其他室壁厚度大于 8 毫米的节段可进行注射,但在基底前壁和下间隔区域需谨慎,以免影响传导系统。
Injection procedure is structured into 3 steps: 1) baseline biplane left ventricular cine-angiography, with co-localization of echocardiographic data for injection guidance, 2) device preparation and introduction into the LV, and 3) navigation and injection.
注射过程分为 3 个步骤:1) 基线双平面左心室电影血管造影,并结合超声心动图数据进行注射引导;2) 器械准备并引入左心室;3) 导航和注射操作。
Contrast left ventriculography is performed in 2 orthogonal views, generally 30+10° right anterior oblique (RAO) and 60+10° left anterior oblique (LAO), modified in each patient to maximize visualization of the endocardium and to foreshorten the interventricular septum. The frozen images are then projected.
在两个正交切面上进行左心室造影,一般为 30+10° 右前斜切面 (RAO) 和 60+10° 左前斜切面 (LAO),根据每位患者的具体情况调整,以最大限度地观察心内膜,并缩短室间隔的投影长度。随后投射冻结图像。
The latter is in our experience important in distinguishing septal and lateral walls, as well as in confirming C-Cathez® location for demarcation of injections.
根据我们的经验,后者对于区分室间隔和侧壁以及确认 C-Cathez® 的注射位置非常重要。
Freeze-frame end-diastolic images are chosen to generate silhouettes marked onto a transparent plastic overlay, onto which target and non-target regions from echocardiography are co-registered.
选择舒张末期的冻结图像以生成轮廓,在透明塑料覆膜上描绘出轮廓,并将超声心动图中的目标区域和非目标区域一起标记好。
The outline of the distal aspect of the pigtail catheter served as an internal linear reference. As the distal tips of most pigtail catheters are circular with diameters of 1-1.5 cm, this was measured and used as an approximation of the recommended inter-injection spacing, and was also marked on each transparency.
猪尾导管远端的轮廓作为内部线性参考。由于大多数猪尾导管的远端头端呈 1 至 1.5 厘米直径的圆形,因此对其直径进行测量,并作为推荐的注射间距的近似值使用,同时在每张透明覆膜上也做了标记。
The pre-procedure preparation of C-Cathez® contains irrigation of the injection lumen with heparinized saline, evaluation of the device’s deflection and setting the needle length with the device in both extended and 90°-deflected positions were made.
C-Cathez® 术前准备包括用肝素化生理盐水冲洗注射腔,评估器械调弯情况,并在器械处于伸直和 90° 弯曲两种状态下设定针头长度。
Needle length is adjusted to correspond to 50% of the wall thicknesses measured on echocardiogram. With a 1 mL Luer-lock syringe connected to the proximal port, the needle’s 0.1 mL dead space was irrigated with heparinized saline.
针头长度根据超声心动图测量的心室壁厚度的 50% 进行调整。将 1 毫升鲁尔锁注射器连接到近端端口,用肝素化生理盐水冲洗针头的 0.1 毫升死腔。
Once prepared and primed with cells, the catheter is inserted via the femoral artery and advanced into the left ventricle in retro-aortic fashion.
细胞经准备和处理后,导管通过股动脉插入,以主动脉逆行的方式推进至左心室。
Long sheath is advised to minimize vascular trauma on retrograde passage of the device, to minimize the need for table repositioning.
建议使用长鞘,以减少器械逆行通过时对血管的损伤,并最小化手术台重新定位的需求。
Aortic valve is to be crossed in least atraumatic manner with catheter flexed to apply a 180° curve in the ascending aorta and maneuver the C-Cathez® across the aortic valve curve first.
以最不损伤的方式跨主动脉瓣,弯曲导管,在升主动脉上形成 180° 的弧度,并先操控 C-Cathez® 穿过主动脉瓣曲线。
In the LV chamber, the deflection is then removed and the device tip placed at mid-cavity level in the “neutral position”.
然后,在左心室中松弯,并将器械头端置于心腔中部水平的 "中立位置"。
With landmarks viewed on real-time fluoroscopy aligned with those inscribed on transparencies, the device can be maneuvered to the pre-determined sites of desired injection sites.
将实时透视图像中的标志与透明覆膜上的标志对齐,可以将器械操纵到预先确定的所需注射部位。
Alternative strategies for delivery were performed using 3D LV angiography with utilizing similar segmentation as described above.
其他的策略采用了三维左心室血管造影术,利用上述类似的分割技术实施递送。
# Summary
In all catheters, the injection strategy and target areas should be optimally planned in advance using all available clinical information along with the necessary imaging modalities.
对于所有导管,都应事先利用所有可用的临床信息和必要的成像模式,对注射策略和目标区域进行最佳规划。
The selection of the individual catheter and the size should be carefully chosen and periprocedural safety monitoring is mandatory.
应谨慎选择单独的导管和尺寸,并且必须进行围手术期安全监测。
The injection technique is similar for catheters with manually retractable needles such as C-Cathez®, MyoCath™ or MyoStar™.
对于带有手动可伸缩针头的导管(例如 C-Cathez®、MyoCath™ 或 MyoStar™),注射技术类似。
The needle extension is performed swiftly after having made stable and perpendicular contact with the vessel wall as documented under fluoroscopic or electromechanical guidance.
在透视或机电引导下,针头与血管壁稳定垂直接触后,迅速进行针头延伸。
The injection of the biologics should be performed slowly to allow for the fluid absorption by the myocardium. The volume varies between 0.1 mL and 0.5 mL per injection with maximum of biologics up to 10 mL in some studies.
生物制剂的注射应缓慢进行,以便心肌能够吸收液体。每次注射量在 0.1 毫升到 0.5 毫升之间,有些研究发现生物制剂的最大注射量可达 10 毫升。
After delivery of the designated volume per injection, the needle should remain settled for few seconds and retracted with some delay to prevent backflow and expulsion of the biologics due to myocardial squeezing.
每次注射指定剂量后,针头应静置数秒,并稍延时回撤,以防心肌挤压导致的生物制剂回流和排出。
In all cases, post-procedural monitoring is comparable to other interventional procedures, including a recommended monitoring of cardiac enzymes.
在所有情况下,术后监测与其他介入手术类似,包括建议的心肌酶监测。
# FOCUS BOX 3
Direct intramyocardial cell delivery 直接心肌内细胞递送
- Percutaneous endoventricular delivery, as a standalone procedure, can be used in ischaemic heart failure under fluoroscopic or electromechanical guidance.
在透视或电机械引导下,经皮心内膜递送作为一种独立手术,可用于缺血性心力衰竭 - Following injection catheters have been used so far in clinical trials of cell delivery:
目前临床试验中用于细胞递送的注射导管包括:
the Helix™ infusion catheter (BioCardia Inc., San Francisco, CA, USA) with a helical needle and MyoCath™ (Bioheart Inc.) and MyoStar™ (Biologics Delivery Systems) with a straight needle and C-Cathez (Celyad, Mont-Saint Guibert, Belgium with curve needle tip with side holes.
带有螺旋针头的 Helix™输液导管,带有直针头的 MyoCath™和 MyoStar™,以及带有侧孔的弯针头的 C-Cathez。 - The injection strategy and target areas should be well planned in advance using the available clinical information
应利用现有的临床信息,提前规划好注射策略和目标区域 - Injections should be performed by a dedicated team after prior training. The needle extension must be performed swiftly after having established stable and perpendicular contact with the vessel wall as documented under fluoroscopic or electromechanical guidance. The injection of the biologics should be performed slowly to allow for the fluid absorption by the myocardium.
注射应由经过事先培训的专门团队进行。必须在透视或电机械引导下,与血管壁建立稳定且垂直的接触,然后迅速出针。生物制剂的注射应缓慢进行,以便心肌吸收液体。
# Delivery of biologics and hurdles in clinical translation 生物制品的递送及临床转化中的障碍
Durable cell retention remains the Achilles heel of biologic based regeneration therapy.
细胞的持久保留仍然是生物再生疗法的致命弱点。
In comparison studies, the utilization of endomyocardial delivery techniques consistently reveals higher rates of cell retention [24] , [25]. By association, optimizing endomyocardial delivery systems should impact clinical outcomes, the ultimate aim of regenerative biologic therapy.
在对比研究中,利用心内膜递送技术始终显示出更高的细胞保留率。由此可见,优化心内膜递送系统应当能影响临床结果,这也是再生生物疗法的最终目标。
Accordingly, in the largest and most detailed experience with intramyocardial injections from the CHART-1 trial suggests potential benefits in a targeted patient selection of ischemic heart failure with optimal treatment intensity for given delivery device [26].
因此,CHART-1 试验中最大规模、最详细的心肌内注射经验表明,有针对性地选择缺血性心力衰竭患者,并根据给药装置选择最佳治疗强度,可能会带来潜在的益处。
While initial short term outcomes remained neutral in this trial [27] longer term follow up analysis highlights possible favorable outcomes in patients with an left ventricular end diastolic volume of 200-370mL [26].
虽然这项试验的最初短期结果保持中立,但长期随访分析显示,左心室舒张末期容积为 200-370 毫升的患者可能获得良好的治疗效果。
The risks of cardiovascular (CV) hospitalizations and a composite of CV hospitalization and death were lower in patients treated with biologic cell therapy.
接受生物细胞疗法治疗的患者,心血管疾病住院风险以及心血管疾病住院和死亡的综合风险较低。
The avenues for the future development include the biology of the myocardial niche-cell and device-niche interaction [6] , [7] (Figure 5).
未来的发展方向包括,心肌微环境 - 细胞生物学,及医疗器械 - 微环境的交互作用(图 5)。
Myocardial niche-cell:心肌微环境中的细胞,可能指的是心肌细胞及其周围的支持细胞(如间质细胞、内皮细胞等)。这些细胞在心脏的功能、修复和再生中起重要作用。
Device-niche interaction:指的是医疗设备(如心脏支架、起搏器等)与心肌微环境之间的相互作用。这种相互作用可能影响设备的性能、组织的愈合以及整体的治疗效果。
With regard to niche-cell interaction, chemoattractive forces are generally favourable for cells to adhere to vascular or structural myocardial compartments early after acute ischaemic injury.
关于微环境 - 细胞相互作用,化学吸引力通常有利于细胞在急性缺血性损伤后早期附着于血管或心肌结构区。
化学吸引力(chemoattractive forces):是指细胞朝向较高浓度的化学信号(如趋化因子)移动的现象。这些信号通常是在损伤后由受损组织释放的,帮助引导细胞到达需要修复的区域。
Debate perists as to whether these signals are present in the setting of remodelled and chronically dysfunctional myocardium.
关于这些信号是否存在于重塑和慢性功能障碍的心肌环境中,目前还存在争议。
Our continuously improving insight of the signaling profiles of diseased myocardium relative to the extent of remodeling and dysfunction [14] is important in predicting the success of cell adhesion and extent of migration likely to occur after stem cell infusion.
我们对病变心肌的信号特征与重塑和功能障碍程度的了解不断加深,这对预测干细胞输注后细胞粘附的成功率和可能发生的迁移程度非常重要。
Aligned to this, efforts aimed at priming the targeted tissue for more efficacious retention can be established.
与此相一致,为更有效地留住细胞,可以对目标组织进行引诱。
Alternative strategies to augment cell retention such as by the pharmacological priming of the cells using genetic modifications or the use of resorbable scaffolds are being investigated.
目前正在研究增强细胞存留的其他策略,如利用基因修饰对细胞进行药理诱导,或使用可吸收支架。
Besides enhancing the immediate engraftment, these strategies are also relevant with regard to improved cell survival, a major prerequisite for any sustained therapeutic benefit.
除了加强直接移植外,这些策略还与提高细胞存活率有关,而细胞存活率是获得持续治疗效果的重要前提。
Likewise, when considering the extent of the negative remodelling, including wall thinning, excessive fibrosis replacement integration of “non-cellular” components with biologics is likely to be critical to balance off a number of factors which adversely affect cell survival and retention.
同样,在考虑负性重塑的程度(包括室壁变薄、过度纤维化替代)时,将 "非细胞" 成分与生物制剂结合起来,对于平衡对细胞存活和存留产生不利影响的一系列因素可能至关重要。
Cell scaffolding alone or in combination with various self-assembling matrices used as a delivery platform may add not only to the mechanistic support of cells or thinned tissue, but also could be helpful to enhance cell maturation by applying a multidimensional spatial relationship or by serving as a carrier of nutrients.
细胞支架单独使用或与各种自组装基质结合使用作为递送平台,不仅可以增加对细胞或变薄组织的机械支持,还可以通过应用多维空间关系或作为营养物质的载体,来帮助提高细胞成熟度。
In the setting of chronically ischemic myocardium, tissue heterogeneity due to fibrosis and non-viable myocardium profoundly negate cell-tissue-device interaction.
在心肌长期缺血的情况下,纤维化和非存活心肌造成的组织异质性,会严重影响细胞 - 组织 - 器械之间的相互作用。
The interplay between these components is variable and dependent on their unique properties and it is for the delivery system to assure effective administration of biologics with minimal trauma to cells or tissue.
这些成分之间的相互作用是多变的,并取决于它们的独特性质,而给药系统则要确保生物制剂的有效给药,同时尽量减少对细胞或组织的创伤。
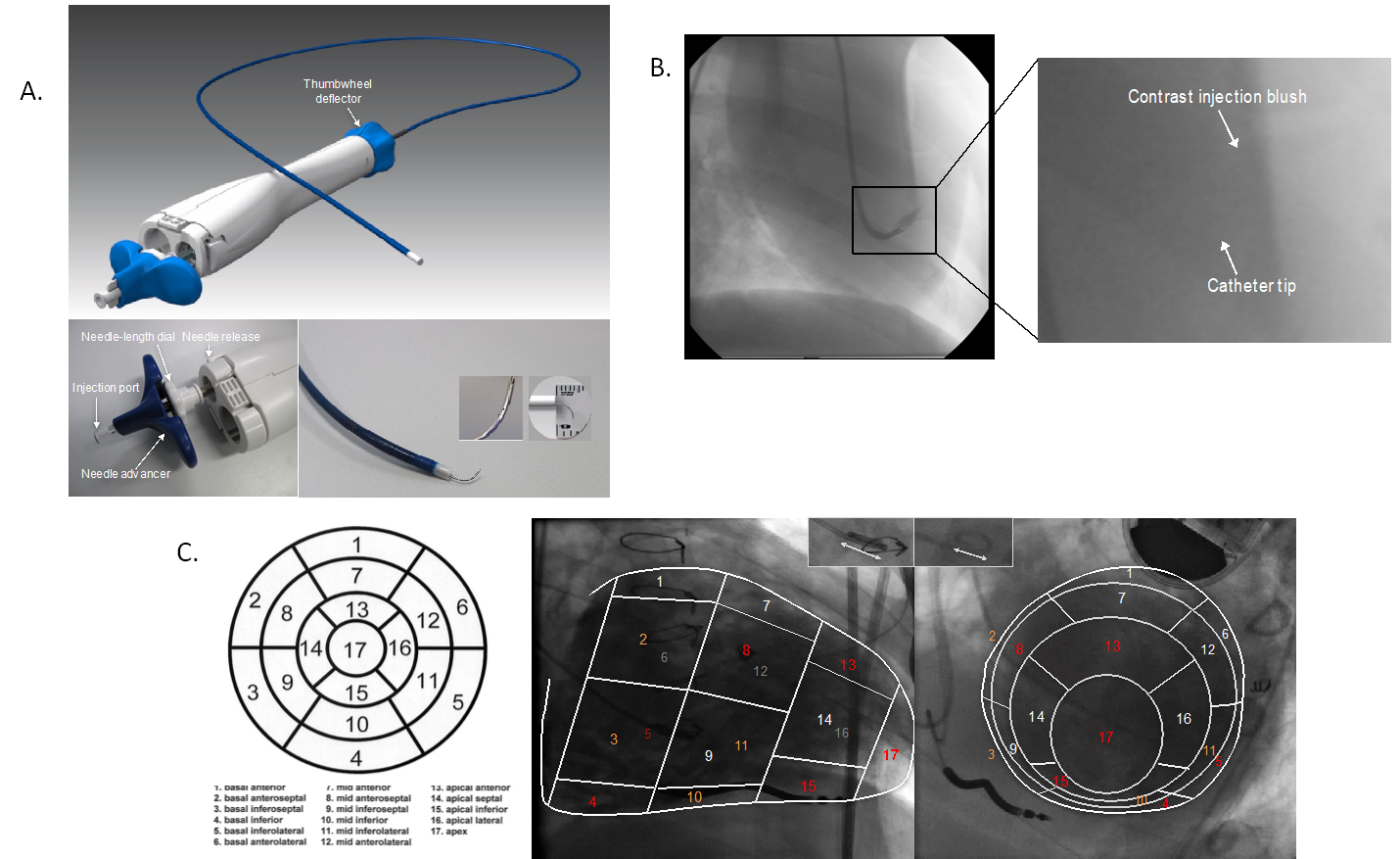
In this regard, mathematical modelling incorporating key mechanical facets of cell-tissue-device interaction appeared to help to predict the spatio-temporal dynamics following myocardial cell delivery (Figure 6) and defined the optimal needle design as showcased by C-CathTM.
在这方面,结合细胞 - 组织 - 器械交互关键力学特征的数学建模,似乎有助于预测心肌细胞递送后的时空动态变化(图 6),并明确了 C-Cath 所展示的最佳针头设计。
Mathematical modelling of a needle design depicting cell dispersion with a curved design and side holes (upper panel).
针头设计的数学模型,描绘了带有弯曲设计和侧孔的细胞分散情况(上图)。
Contrast injection using a catheter prototype with a curved needle and side holes confirms the modelled situation in vivo with dispersed contrast injection around the side holes and tip of the needle (lower panel).
使用带有弯曲针头和侧孔的导管原型进行造影剂注射,证实了体内的模拟情况,即造影剂在侧孔和针尖周围分散注射(下图)。
(Image courtesy of Cardio3 Biosciences, Mont St-Guibert, Belgium)
Likewise, in the context of cell-tissue-device interaction, better understanding is needed to define the optimal relationship between the dose, concentration and volume of biologics and maximal retention and therapeutic effect.
同样,在细胞 - 组织 - 器械相互作用的背景下,需要更好地理解生物制剂的剂量、浓度和体积与最大保留和治疗效果之间的最佳关系。
It remains unclear what is the optimal cell dose/volume for individual and total number of injections and what number of injections may lead to better functional benefit without increasing the procedural risk.
目前尚不清楚单次和总注射的最佳细胞剂量 / 体积是多少,以及多少次注射可能带来更好的功能益处而不增加手术风险。
This may be critical for a proper definition of the dose-dependent response and answer the question of whether repeat injections to deliver the target dose of the biologics may be appropriate rather than a one-procedure treatment with injection of high dose/high volume biologic.
这对于准确界定剂量依赖性反应至关重要,并能解答是否适合多次注射以达到生物制剂目标剂量,而非一次性高剂量 / 高容量注射的单次治疗方案的问题。
In this regard, an experimental study has indicated that multisite injection with smaller volumes (‘microdeposits’) resulted in superior efficacy as compared to delivery of the same dose but given in the smaller number of injections with large volume [28].
在这方面,实验研究表明,多部位小剂量注射(“微沉积”) 相较于相同剂量但次数较少的大体积注射,具有更优异的效果。
Moreover, the choice of appropriate imaging and guidance to the desired target myocardial areas remains to be further explored. Real time electromechanical guidance is currently the benchmark technology.
此外,如何选择合适的成像技术,并引导至目标心肌区域仍有待进一步探索。实时电机械引导是目前的基准技术。
However, MRI-guided approach has been successfully tested in animal models [29] , [30] and has the capacity to enable disease-specific targeting.
不过,核磁共振成像引导方法已在动物模型中成功进行了测试,并有能力实现特定疾病的靶向治疗。
Advances in imaging technology such as image fusion, intracardiac ultrasound or other imaging modalities allowing 3D orientation, such as CT, are likely to be progressively explored to ensure precision in targeting in the designated myocardial areas.
成像技术的进步,如图像融合、心腔内超声或其他可进行三维定位的成像模式(如 CT),很可能会逐步得到探索,以确保在指定心肌区域实现精准靶向。
# FOCUS BOX 4
Future development of cell delivery techniques 细胞递送技术的未来发展
- Strategies to improve cell delivery and retention include better understanding and exploration of the myocardial niche-cell and device-niche interaction.
提高细胞递送和留存的策略,包括更好地理解和探索心肌微环境 - 细胞及器械 - 微环境之间的相互作用。 - Tissue priming, or cell priming including the use of cell scaffolds, are under testing to improve cell retention and survival.
组织预处理,或包括使用细胞支架在内的细胞预处理,正在测试中,以提高细胞的留存率和存活率。 - Mathematical modelling, hand in hand with experimental in vitro and in vivo testing, is a sound platform to validate various needle designs to improve cell retention.
数学建模,与体外、体内实验测试相辅相成,是验证不同针头设计以提高细胞留存率的可靠平台。
# Conclusions
Advances in stem cell delivery systems must keep pace if progress in cardiovascular regenerative medicine is to be assured.
干细胞递送系统的进步必须与时俱进,以确保心血管再生医学的持续进步。
Development strategies should address the main aspects of delivery in a concerted manner, ensuring that integration of the three components of biologics-based tissue repair (underlying disease, cell preparation and delivery method) is enables biologic-induced regenerative signaling with minimized susceptibility to its attrition (Figure 5) [6] , [7] , [8].
开发策略应协调一致地解决递送的主要方面,确保基于生物制剂的组织修复三要素(基础疾病、细胞制备及递送方式)的整合,能够实现生物制剂诱导的再生信号,并最大限度地降低其损耗(图 5)。
The interplay between these components is variable and dependent on their unique properties and it is for the delivery system to assure effective biologics administration, with minimal trauma to cells or tissue.
这些组成要素之间的相互作用是多变的,并取决于它们的独特性质,因此给药系统必须确保生物制剂的有效给药,同时尽量减少对细胞或组织的创伤。
Importantly, preclinical and clinical development will also depend on the interventional skills of the operators.
重要的是,临床前和临床开发还将取决于术者的介入技能。
Intramyocardial injection catheters function within a different framework than vascular devices and, while a fair body of experience exists, most have seen limited use in clinical trials to date.
心肌内注射导管的工作原理与血管器械不同,尽管积累了一定的临床经验,但迄今为止,大多数导管在临床试验中的应用都很有限。
Even though none are overly demanding in concept or mechanics, learning curves are still being charted for all of them, especially for those in which advanced myocardial disease is being targeted [31].
尽管这些方案在概念或机制上都没有过高的要求,但所有方案的学习曲线仍在绘制之中,尤其是针对晚期心肌疾病的方案。
# Personal perspective - Jozef Bartunek
Cardiac regeneration therapy remains a promising intervention that is based on sound biological concepts and experimental evidence.
心脏再生疗法以合理的生物学概念和实验证据为基础,仍然是一种前景广阔的干预方法。
Though overall clinical evidence points to neutral or modest therapeutic benefits, the basic foundation and advances in the translational stem cell research do hold the promise for developing clinically relevant biologic-based intervention in ischaemic heart failure.
虽然总体临床证据显示治疗效果可能是中性或适度的,但干细胞转化研究的基础和进展,确实为开发针对缺血性心力衰竭的临床相关生物基础干预措施提供了希望。
It is reassuring that randomised controlled clinical trials demonstrated a favourable safety profile with clinically impactful signals of efficacy in well-defined patient population.
令人欣慰的是,随机对照临床试验显示,这种疗法在特定患者群体中具有良好的安全性,并显示出具有临床意义的疗效信号。
While advances in cell biology or non-cellular scaffolds are likely to propel further the regenerative strategies, cell delivery will need to follow.
虽然细胞生物学或非细胞支架的进步可能会进一步推动再生策略,但细胞递送也需要跟上。
The pharmacologically effective and consistent delivery is essential to ensure target tissue biological and functional repair to maximise the potential of various biologics.
药理学上有效且持续的递送,对于实现各种生物制剂的潜能,促进靶组织生物和功能修复至关重要。
Further development should focus on device and procedural development to increase the immediate retention without additional myocardial damage.
进一步发展的重点应放在器械和术式的开发上,以提高即时留存率,而不会对心肌造成额外的损伤。
The targeting and guidance of cell delivery is also suboptimal.
细胞递送的靶向性和引导性也不够理想。
New imaging modalities such as MRI, CT or 3D echocardiography or angiography and their combination in the fusion imaging are being tested as alternative strategies.
正在测试新的成像方式,如核磁共振成像、CT 或三维超声心动图或血管造影,以及它们在融合成像中的组合,作为替代策略。
We are also falling short in understanding the optimal relationship between the dose, concentration and volume of biologics needed for the maximal therapeutic effect.
我们对于实现最大治疗效果所需生物制剂的最佳剂量、浓度与体积之间的关系仍理解不足。
It remains unclear what is the optimal cell dose/volume for individual or total number of injections, what number of injections may lead to better functional benefit without increasing the procedural risk.
目前还不清楚最佳的细胞剂量 / 体积是针对个体还是总注射次数,也不知道多少次注射可以在不增加手术风险的情况下带来更好的功能效益。
This is critical for the design of future clinical trials for the proper definition of the dose-dependent response as well as the question as to whether repeat injections to deliver the target dose of the biologics may be suitable rather than one-procedure treatment with injection of high dose/high volume biologic.
这对于设计未来的临床试验至关重要,以便正确定义剂量依赖性反应,以及是否适合通过重复注射来提供目标剂量的生物制剂,而不是注射高剂量 / 大体积生物制剂的一次性治疗。
# References
Abdel-Lattif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989-97.Link ↩︎ ↩︎ ↩︎
Lipinski MJ, Biondi-Zoccia GG, Abatte A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761-7.Link ↩︎ ↩︎ ↩︎
Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208-16.Link ↩︎ ↩︎
Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart. 2010;96:792-800.Link ↩︎ ↩︎
Satsuki Y, Bartunek J, Behfar A, Terzic A. Mass Customized Outlook for Regenerative Heart Failure Care. Int J Mol Sci. 2021; 22: 11394.Link ↩︎ ↩︎ ↩︎
Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Prac Cardiovasc Med. 2006;3:Suppl 1:S57-64.Link ↩︎ ↩︎ ↩︎
Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine._ Clin Pharmacol Ther_. 2009;85:548-52.Link ↩︎ ↩︎ ↩︎
Dib N, Khawaja H, Varner S, McCarthy M, Cambell. Cell Therapy for cardiovascular disease: A comparison of methods of delivery. J of Cardiovasc Trans Res. 2011; 4:177-81.Link ↩︎ ↩︎
Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198-202.Link ↩︎ ↩︎
Blocklett D, Toungouz M, Berkenboom G, Lambermont M, Unger P, Preumont N, Stoupel E, Egrise D, Degaute JP, Goldman M, Goldman S. Myocardial homing of nonmobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells. 2006;24:333-6.Link ↩︎
Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47:1295-301.Link ↩︎ ↩︎
Schots R, De Keulenaer G, Schoors D, Caveliers V, Dujardin M, Verheye S, Van Camp G, Franken PR, Roland J, Van Riet I, Everaert H. Evidence that intracoronary-injected CD133+ peripheral blood progenitor cells home to the myocardium in chronic postinfarction heart failure. Exp Hematol. 2007;35:184-9.Link ↩︎
Penicka M, Lang O, Widimsky P, Kobylka P, Kozak T, Vanek T, Dvorak J, Tintera J, Bartunek J. One-day kinetics of myocardial engraftment after intracoronary injection of bone marrow mononuclear cells in patients with acute and chronic myocardial infarction. Heart. 2007;93:837-41.Link ↩︎ ↩︎
Schächinger V, Aicher A, Döbert N, Röver R, Diener J, Fichtischerer S, Assmus B, Seeger FH, Menzel C, Brenner W, Dimmeler S, Zeiher AM. Pilot trial on determinants of progenitor cell recruitment to the infarcted myocardium. Circulation. 2008;118:1425-32.Link ↩︎ ↩︎ ↩︎
Bartunek J, Wijns W, Heyndrickx G, Vanderheyden M. Timing of intracoronary bone-marrow-derived stem cell transplantation after ST-elevation myocardial infarction. Nat Clin Prac Cardiovasc Med. 2006;Suppl 1:S52-56.Link ↩︎
Doyle B, Kemp BJ, Chareonthaitawee P, Reed C, Schmeckpeper J, Sorajja P, Russell S, Araoz P, Riederer SJ, Caplice NM. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med. 2007;48:1708-1714.Link ↩︎
Penn, M, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn F, Strasser D, Ting A, Sherman W. Adventitial Delivery of an Allogenic Bone Marrow-Derived Adherent Stem Cell in Acute Myocardial Infarction. Circulation Research. 2012;110:304-311.Link ↩︎
Suzuki K, Murtuza B, Fukushima S, Smolenski RT, Varela-Carver A, Coppen SR, Yacoub MH. Targeted cell delivery into infarcted rat hearts by retrograde intracoronary infusion: distribution, dynamics, and influence on cardiac function. Circulation. 2004;110:II225-II230.Link ↩︎
Murad-Netto S, Moura R, Romeo LJ, Manoel NA, Duarte N, Barreto F, Jensen A, Vina RF, Vraslovik F, Oberdan A, Benetti F, Saslavsky J, Vina MF, Amino JG. Stem cell therapy with retrograde coronary perfusion in acute myocardial infarction. A new technique. Arq Bras Cardiol. 2004;83:352-4.Link ↩︎
Silva SA, Sousa ALS, Haddad AF, Azevedo JC, Soares VE, Peixoto CM, Soares AJS, Issa AFC, Felipe LRV, Branco RVC. Autologous bone-marrow mononuclear cell transplantation after acute myocardial infarction: comparison of two delivery techniques. Cell Transplant. 2009; 18:343-353.Link ↩︎ ↩︎
Egred M, Bagnall A, Spyridopoulos I, Purcell I, Das R, Palmer N, Grech E, Jain A, Stone G, Nijveldt R, McAndrew T, Zaman A. Effect of Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) on infarct size in anterior STEMI: PiCSO in ACS study. IJC Heart & Vasculature. 2020; 28: 100526.Link ↩︎
Perin EC, Lopez J. Methods of stem cell delivery in cardiac diseases. Nat Clin Pract Cardiovasc Med. 2006;3:S110-S113.Link ↩︎
Sherman W, Bartunek J, Dolatabadi D, Sanz-Ruiz R, Beleslin B, Wojakowski W, Heyndrickx G, Kimpalou JZ, Waldman S, Laarman J, Seron A, Behfar A. First-in-Human Use of a Retention-Enhanced Catheter for Endomyocardial Cell Delivery. J Am Coll Cardiol Intv. 2018; 11:412-414.Link ↩︎
Hou D, Youssef E, Brinton T, Zhang P, Rogers P, Proce E, Yeung A, Johnstone B, Yock P, March K. Radiolabeled Cell Distribution after Intramyocardial, Intracoronary, and Interstitial Retrograde Coronary Venous Delivery. Circulation. 2005; 112: 150-156.Link ↩︎
Li SH, Lai T, Sun Z, Han M, Moriyama E, Wilson B, Fazel S, Weisel R, Yau T, Wu J, Li RK. Tracking cardiac engraftment and distribution of implanted bone marrow cells: Comparing intra-aortic, intravenous, and intramyocardial delivery. The Journal of Thoracic and Cardiovascular Surgery, 2009; 137:1225-1233.Link ↩︎
Bartunek J, Terzic A, Davison B, Behfar A, Sanz-Ruiz R, Wojakowski W, Sherman W, Heyndrickx G, Metra M, Filippatos G. Cardiopoietic stem cell therapy in ischaemic heart failure: long-term clinical outcomes. ESC Heart Failure. 2020; 7:3345-3354.Link ↩︎ ↩︎
Bartunek J, Terzic A, Davison B, Filippatos G, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W. Cardiopoietic cell therapy for advanced ischaemic heart failure at 39 weeks of the prospective, randomized, double-blind, sham-controlled CHART-1 clinical trial. European Heart Journal. 2017; 38:648-660.Link ↩︎
Ott HC, Kroes R, Bonaros B, Marksteiner R, Margreiter E, Schachner T, Laufer G, Hering S. Intramyocardial microdepot injection increases the efficacy of skeletal myoblast transplantation. Eur J Cardiothorac Surg. 2005;27:1017-21.Link ↩︎
Dick AJ, Guttman MA, Raman VK, Peters DC, Pessanha BS, Hill JM, Smith S, Scott G, McVeigh ER, Lederman RJ. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in swine. Circulation. 2003;108:2899-904.Link ↩︎
Tomkowiak MT, Klein AJ, Vigen KK, Hacker TA, Speidel MA, Van Lysel MS, Raval AN. Targeted Transendocardial Therapeutic Delivery Guided by MRI-X-ray Image Fusion. Catheter Cardiovasc Interv. 2011;78:468-78.Link ↩︎
Dib N, Menasche P, Bartunek JJ, Zeiher AM, Terzic A, Chronos NA, Henry TD, Peters NS, Fernández-Avilés F, Yacoub M, Sanborn TA, Demaria A, Schatz RA, Taylor DA, Fuchs S, Itescu S, Miller LW, Dinsmore JH, Dangas GD, Popma JJ, Hall JL, Holmes DR Jr; International Society for Cardiovascular Translational Research. Recommendations for successful training on methods of delivery of biologics for cardiac regeneration: a report of the International Society for Cardiovascular Translational Research. JACC Cardiovasc Interv. 2010;3:265-75.Link ↩︎