# SUMMARY
The patient selection and procedural planning for transcatheter structural interventions have been facilitated by the extensive use of multislice computed tomography (MSCT) multiplanar reconstruction. This imaging technique allows the identification of the optimal fluoroscopic viewing angles of left- and right-sided heart structures, in particular the aortic, mitral and tricuspid valve complexes. The use of standardized fluoroscopic viewing angles, during transcatheter interventions, can be extremely helpful in technically challenging scenarios (e.g., bifurcation stenting, aortic, mitral and tricuspid valve interventions). The continuous refinement of transcatheter procedural techniques and the emergence of novel transcatheter devices challenge our understanding and applicability of multimodality imagining. Mastering multimodality imaging of the heart chamber anatomy can reduce the procedural time, radiation dose, contrast volume, and complications during transcatheter coronary and structural interventions while providing guidance for the operators. In this chapter we aim to describe the anatomy of the heart with respect to structural interventions and coronary arteries by integrating MSCT, fluoroscopy and echocardiography. By doing so, we also describe the utility of optimal fluoroscopic viewing angles in guiding structural and coronary interventions.
# INTRODUCTION
Traditionally, percutaneous coronary interventions (PCI) are performed using well-recognized anatomical patterns based on familiar fluoroscopic viewing angles. Coronary angiography has indeed an excellent resolution, but its two-dimensional (2D) nature inherently limits its diagnostic accuracy due to foreshortening and overlap of structures [1]. Since the introduction of transcatheter structural heart therapies, cardiologists have become increasingly aware of the importance of understanding anatomical details of cardiac structures under fluoroscopy [2]. Many critical anatomical structures comprise several spatial components arranged in a complex three-dimensional (3D) geometry. Even though these anatomical and functional components have been the topic of numerous publications [3], little consideration has been given to understanding their configuration as appreciated under fluoroscopy [4]. Understanding fluoroscopic cardiac anatomy can facilitate optimal positioning and deployment of prostheses during transcatheter valve repair/replacement, left atrial appendage occlusion, septal defect closure, paravalvular leak closure. Commonly, these therapies are conducted using standard fluoroscopic angulations irrespective of variations in anatomy. However, it is possible that patient-specific fluoroscopic viewing angles can improve procedural safety and efficacy.
Interestingly, the common chamber views appreciated on echocardiography can be appreciated across imaging modalities, including fluoroscopy, thereby providing the basis for a common language (i.e. imaging modality independent). Furthermore, optimal projection curves (i.e. S-curves) that provide fluoroscopic viewing angles of a structure in-plane or en-face can be interrelated with fluoroscopic chamber views. Understanding the relationship between chamber views and optimal projection curves is the fundamental principle in mastering “fluoroscopic anatomy” [5].
MSCT multiplanar reconstruction of the aortic valvular complex has enhanced patient selection and procedural planning for transcatheter aortic valve implantation [6]. For example, MSCT affords physicians the opportunity to pre-select optimal x-ray fluoroscopic viewing angles for deployment of the valve prosthesis [7]. Such angle optimization may decrease procedure time, radiation exposure, and injected contrast agent volume [8]. It may reduce the risk of acute kidney injury [9] and paravalvular regurgitation [10], conduction disturbances or valve embolization. The same principles can be applied for planning of any fluoroscopy-guided procedure, including coronary interventions.
# BRIDGING BETWEEN CARDIAC IMAGING MODALITIES: IN SEARCH FOR A COMMON LANGUAGE
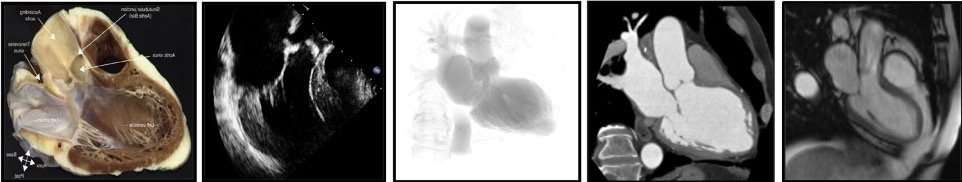
The use of a particular terminology to describe the heart impacts the way physicians describe the structures of the heart. Distinct anatomical terminologies are used by imagers using different imaging modalities. These dissimilarities are often experienced as a major obstacle during procedural planning and realization. For instance, the mid-esophageal mitral bicommissural view on transeophageal echocardiography, the horizontal long-axis view on nuclear cardiac imaging and the right anterior oblique (RAO)/cranial (CRA) projection on fluoroscopy all depict a two-chamber view of the left heart. In fact, the way the heart appears is a matter of viewing angle rather than imaging modality. A three-chamber view of the left heart, showing the left atrium, left ventricle and left ventricular outflow tract (LVOT)/aorta, can be obtained from an anatomical preparation of the heart as well as by echocardiography, fluoroscopy, MSCT or magnetic resonance imaging as long as the heart is considered from a similar angle (Figure 1). The use of a common “anatomy-centric” terminology, rather than “modality-centric” terminology, is thus mandatory to bridge the gaps across imaging techniques. The following principles may pave the way to a common multimodality terminology that will allow for an easier and more efficient interaction between imagers and operators in the catheterization laboratory.
# ATTITUDINAL DESCRIPTION OF HEART ANATOMY
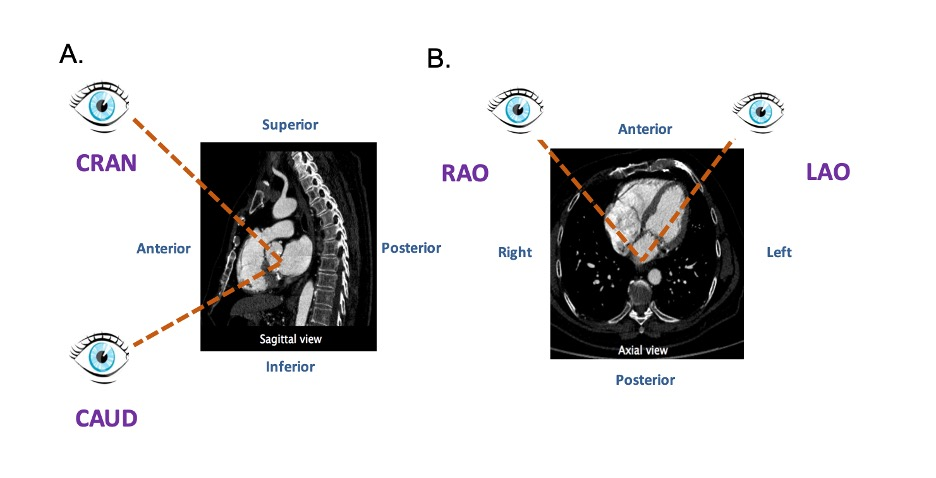
In our discussion, structures will be termed according to their attitudinally correct anatomical position [11]. This implies that the subject is facing the observer and standing upright. Thus, structures closer to the observer are described as being anterior and those relatively farther away within the body are posterior. Components lying closer to the head are superior (i.e., CRA) and those toward the feet are said to be inferior (i.e., caudal [CAU]). Structures to the left-hand side of the observer are right-sided and those to the observer’s right are left-sided (Figure 2).

The fluoroscopic screen portrays the thorax in an upright orientation despite the patient being in a supine position. Superior and inferior structures are appreciated in the upper and lower halves of the screen. The direction of fluoroscopic projections is described based on 2 conventional angles, CRA/CAU and left anterior oblique (LAO)/RAO (Figure 3 A and Figure 3 B). In the anteroposterior (AP) viewing angle (CRA/CAU 0, LAO/RAO 0), right- and left-sided structures are found on the left and right sides of the screen, respectively. Of note, discussing heart structures in their attitudinal position is in perfect agreement with nomenclatures used for MSCT, x-ray fluoroscopic imaging and magnetic resonance imaging (Figure 4); this is not necessarily true with echocardiography.
![Figure 3 - Geometry of the Optimal Projection Curve. The vector joining the x-ray source and the center point of the detector is designated Vd, and the vector pointing along a structure of interest is Vs. (A and B) The angular system (cranial [CRA]/caudal [CAU] and right anterior oblique [RAO]/left anterior oblique [LAO] angles) used in fluoroscopy is described. (C) All vectors Vd perpendicular to vector vs are optimal viewing angles. The optimal projection curve is the plot of the fluoroscopic angles of all vectors Vd for a particular structure of interest. (D) A typical S-shaped optimal projection curve for a given cardiac structure is shown. Figure 3](https://s2.loli.net/2023/11/13/7jpMXgr32DJon1v.png)
Di Mario C, Sutaria N. Coronary angiography in the angioplasty era: projections with a meaning. Heart. 2005 Jul;91(7):968–76. ↩︎
Van Mieghem NM, Piazza N, Anderson RH, Tzikas A, Nieman K, De Laat LE, McGhie JS, Geleijnse ML, Feldman T, Serruys PW, de Jaegere PP. Anatomy of the mitral valvular complex and its implications for transcatheter interventions for mitral regurgitation. J Am Coll Cardiol. 2010 Aug;56(8):617–26. ↩︎
Cosío FG, Anderson RH, Kuck KH, Becker A, Borggrefe M, Campbell RW, Gaita F, Guiraudon GM, Haïssaguerre M, Rufilanchas JJ, Thiene G, Wellens HJ, Langberg J, Benditt DG, Bharati S, Klein G, Marchlinski F, Saksena S. Living anatomy of the atrioventricular junctions. A guide to electrophysiologic mapping. A Consensus Statement from the Cardiac Nomenclature Study Group, Working Group of Arrhythmias, European Society of Cardiology, and the Task Force on Cardiac Nomenclature from NASPE. Circulation. 1999 Aug;100(5):e31-7. ↩︎
Thériault-Lauzier P, Andalib A, Martucci G, Mylotte D, Cecere R, Lange R, Tchétché D, Modine T, van Mieghem N, Windecker S, Buithieu J, Piazza N. Fluoroscopic anatomy of left-sided heart structures for transcatheter interventions: insight from multislice computed tomography. JACC Cardiovasc Interv. 2014 Sep;7(9):947–57. ↩︎
Pighi M, Thériault-Lauzier P, Alosaimi H, Spaziano M, Martucci G, Xiong TY, Buithieu J, Ybarra LF, Afilalo J, Leipsic J, Ozden Tok O, Mousavi N, Mangiameli A, Pilgrim T, Praz F, Windecker S, Piazza N. Fluoroscopic Anatomy of Right-Sided Heart Structures for Transcatheter Interventions. JACC Cardiovasc Interv. 2018 Aug;11(16):1614–25. ↩︎
Spaziano M, Thériault-Lauzier P, Meti N, Vaquerizo B, Blanke P, Deli-Hussein J, Chetrit M, Galatos C, Buithieu J, Lange R, Martucci G, Leipsic J, Piazza N. Optimal fluoroscopic viewing angles of left-sided heart structures in patients with aortic stenosis and mitral regurgitation based on multislice computed tomography. J Cardiovasc Comput Tomogr. 2016;10(2):162–72. ↩︎
Schultz C, Moelker A, Tzikas A, Piazza N, de Feyter P, van Geuns RJ, Serruys PW, Krestin GP, de Jaegere P. The use of MSCT for the evaluation of the aortic root before transcutaneous aortic valve implantation: the Rotterdam approach. EuroIntervention J Eur Collab with Work Gr Interv Cardiol Eur Soc Cardiol. 2010 Sep;6(4):505–11. ↩︎
Dvir D, Kornowski R. Percutaneous aortic valve implantation using novel imaging guidance. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2010 Sep;76(3):450–4. ↩︎
Samim M, Stella PR, Agostoni P, Kluin J, Ramjankhan F, Budde RPJ, Sieswerda G, Algeri E, van Belle C, Elkalioubie A, Juthier F, Belkacemi A, Bertrand ME, Doevendans PA, Van Belle E. Automated 3D analysis of pre-procedural MDCT to predict annulus plane angulation and C-arm positioning: benefit on procedural outcome in patients referred for TAVR. JACC Cardiovasc Imaging. 2013 Feb;6(2):238–48. ↩︎
Poon KK, Crowhurst J, James C, Campbell D, Roper D, Chan J, Incani A, Clarke A, Tesar P, Aroney C, Raffel OC, Walters DL. Impact of optimising fluoroscopic implant angles on paravalvular regurgitation in transcatheter aortic valve replacements - utility of three-dimensional rotational angiography. EuroIntervention J Eur Collab with Work Gr Interv Cardiol Eur Soc Cardiol. 2012 Sep;8(5):538–45. ↩︎
Zgheib Ali, Campens L, Abualsaud Ali, Abdullah Al Isma’ili, Barbanti M Dvir D, Gada H, Granada J, Latib A, Leipsic J, Maisano F, Martucci G, Medina de Chazal H, Modine T, Mylotte D, Prendergast B, Sawaya F, Spaziano M, Tang G, Theriault-Lauzier P, Tchetche D,van Mieghem N, Söndergaard L, De Backer O, Piazza N. Aortic Annulus S-Curve: Implications for Transcatheter Aortic Valve Replacement and Related Procedures, Part 1. JACC Cardiovasc Interv. 2022 Nov. Ahead of print. ↩︎